Your How many grams is one mole of cu images are available. How many grams is one mole of cu are a topic that is being searched for and liked by netizens today. You can Download the How many grams is one mole of cu files here. Get all free photos and vectors.
If you’re looking for how many grams is one mole of cu images information linked to the how many grams is one mole of cu interest, you have come to the ideal site. Our website frequently gives you suggestions for viewing the maximum quality video and picture content, please kindly surf and find more informative video content and graphics that fit your interests.
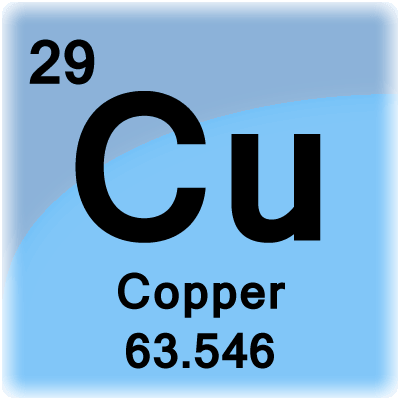
How Many Grams Is One Mole Of Cu. Copper weighs 894 gram per cubic centimeter or 8 940 kilogram per cubic meter ie. 0094 moles of Cu. There fore since the number of atoms in 1 mole is 6022 x 1023 atoms per mole. The SI base unit for amount of substance is the mole.
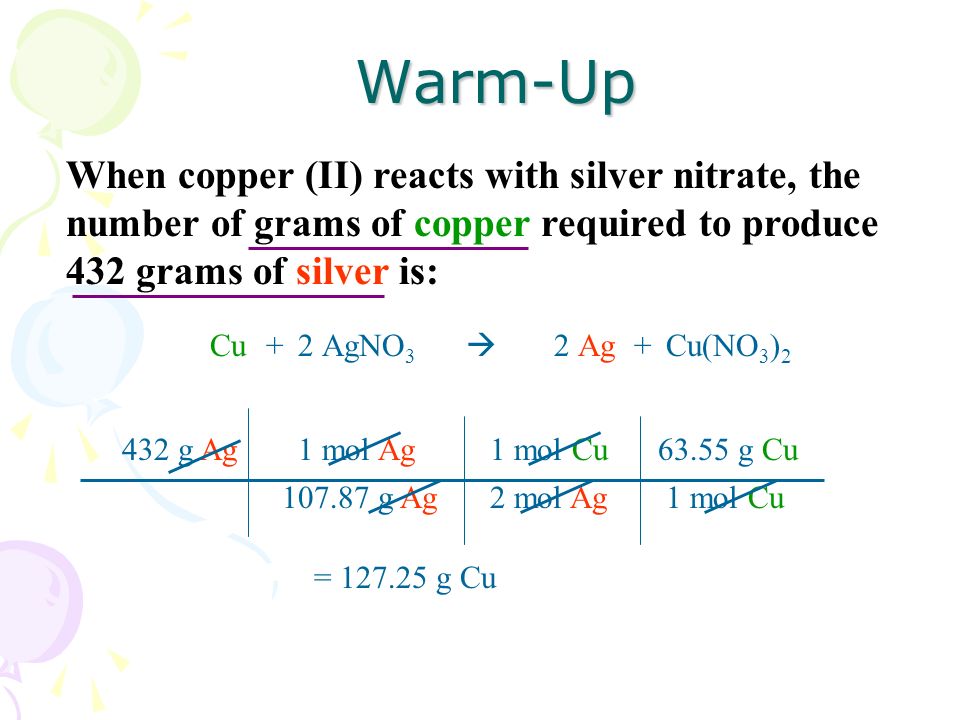 When Copper Ii Reacts With Silver Nitrate The Number Of Grams Of Copper Required To Produce 432 Grams Of Silver Is Warm Up Cuagno 3 Ag22 Cu No 3 Ppt Download From slideplayer.com
When Copper Ii Reacts With Silver Nitrate The Number Of Grams Of Copper Required To Produce 432 Grams Of Silver Is Warm Up Cuagno 3 Ag22 Cu No 3 Ppt Download From slideplayer.com
So you have 253 g CuSO4. How many moles Copper in 1 grams. 1 mole is equal to 1 moles CuSO4 5H2O or 249685 grams. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. This is where you are missing some information. 1 mole of copper 602 10 23 copper atoms.
The symbol of mole is mol.
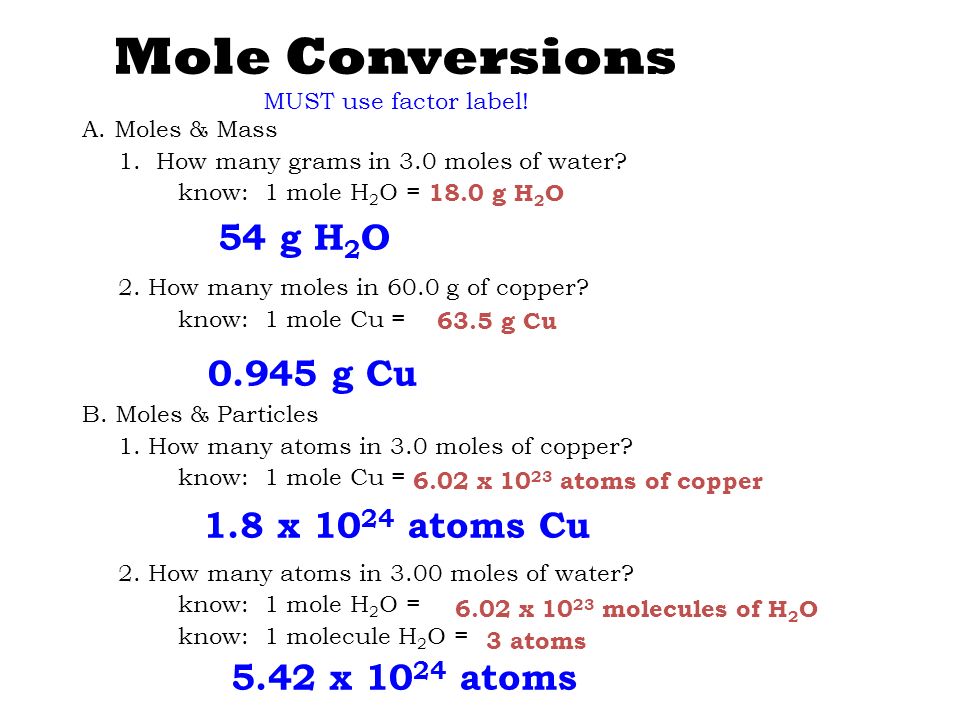
You can view more details on each measurement unit. From your Periodic Table we learn that one mole of copper 60221023 individual copper atoms have a mass of 6355g. C One mole of a monatomic element has a mass equal to its atomic mass expressed in grams. 1 mole is equal to 1 moles CuSO4 or 1596086 grams. The answer is rounded to three sig figs the number of sig figs you have for the number of moles of copper II phosphate. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS.
 Source: nl.pinterest.com
Source: nl.pinterest.com
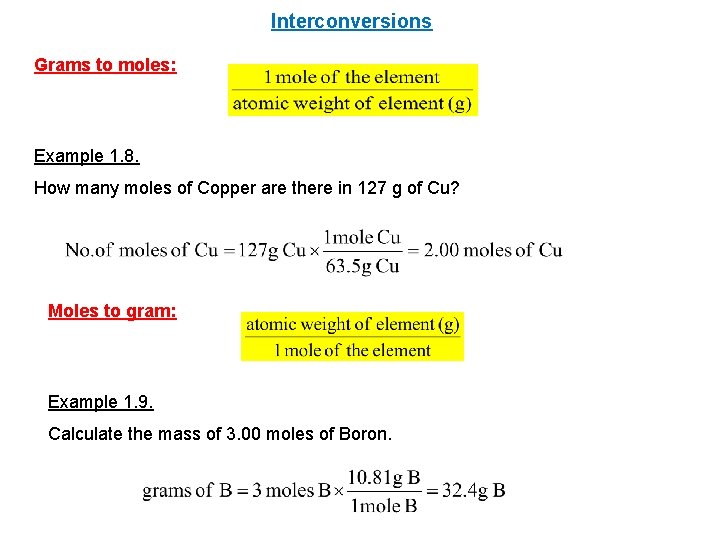
Explore more on it. The Concept Of Mole By Padge Joseph Chemistry Classroom Chemistry Lessons Teaching Chemistry _____grams How Many Moles Of CopperII Sulfite Are Present In 161 Grams Of This Compound. The total number of atoms in a substance can also be determined by using the relationship between grams moles and atoms. A One mole of atoms makes up an amount of atoms that can be seen with the naked eye. At 20C 68F or 29315K at standard atmospheric pressure.
 Source: slidetodoc.com
Source: slidetodoc.com
The molecular formula for Sodium Hydroxide is NaOH. So you have 253 g CuSO4. 63546 x 1 32066 x 1 159994 x 4 10079 x. Before you can find the mass what do you need to know. Keeping this in consideration how do you calculate moles of CuSO4.
 Source: slideplayer.com
Source: slideplayer.com
10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS. 0094 moles of Cu. How many moles are in 1 mole of copper. How do you find the moles of copper sulfate. How many moles Copper in 1 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
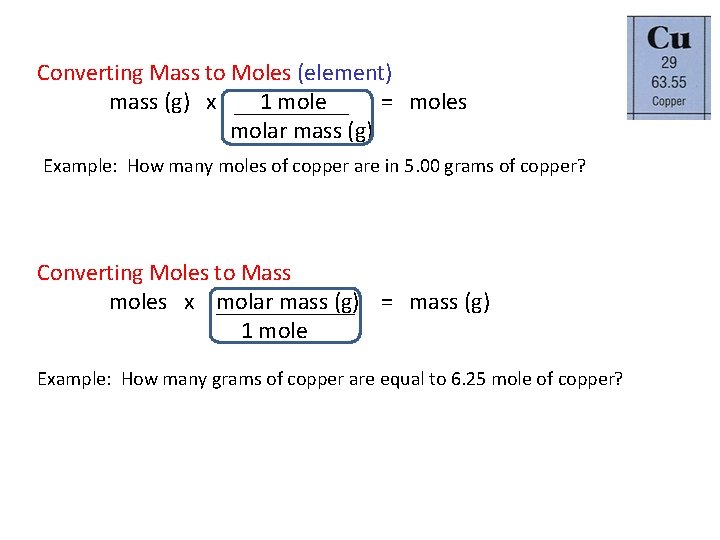
One thing any chemistry professor will tell you is to always GO TO MOLES first. D One mole of water contains 12 mole of oxygen atoms. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. Number of moles in 6 grams 6g x 1 mole 63546g. So copper II sulfate pentahydrate has a molar mass of.
 Source: slidetodoc.com
Source: slidetodoc.com
6022 x 10 23. 0094 moles of Cu. How many moles are in 1 gram of copper. How many moles are equal to 625g of copper. The molecular formula for Sodium Hydroxide is NaOH.
 Source: socratic.org
Source: socratic.org
This means 1 mole of CU atoms has a mass of 63546 grams. Note that rounding errors may occur so always check the results. 225 x 1025 atoms of lead 1 mole 2072g 774419g 7740g 602 x 1023 atoms 1 mole 2. Convert this to moles by the use of the molecular weight. 6022 x 10 23.
 Source: slidetodoc.com
Source: slidetodoc.com
6022 x 10 23. The CuSO4 molecule is made up of six atoms one copper one sulfur and four oxygen. Avogadros Number 1 mole 6022 1023particles and the relative atomic mass of copper 1 mole of copper 63546 g are the equalities required to make this conversion. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. 1 mole is equal to 1 moles CuSO4 or 1596086 grams.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between moles Copper and gram. Keeping this in consideration how do you calculate moles of CuSO4. 1 mole is equal to 1 moles Copper or. The molecular formula for Sodium Hydroxide is NaOH. 1 mole is equal to 1 moles CuSO4 5H2O or 249685 grams.
 Source: youtube.com
Source: youtube.com
So you have 253 g CuSO4. 5988 kg 5988 g. The molar mass of Cu 63546 grams per mole. The answer is 0015736631731344. There fore since the number of atoms in 1 mole is 6022 x 1023 atoms per mole.
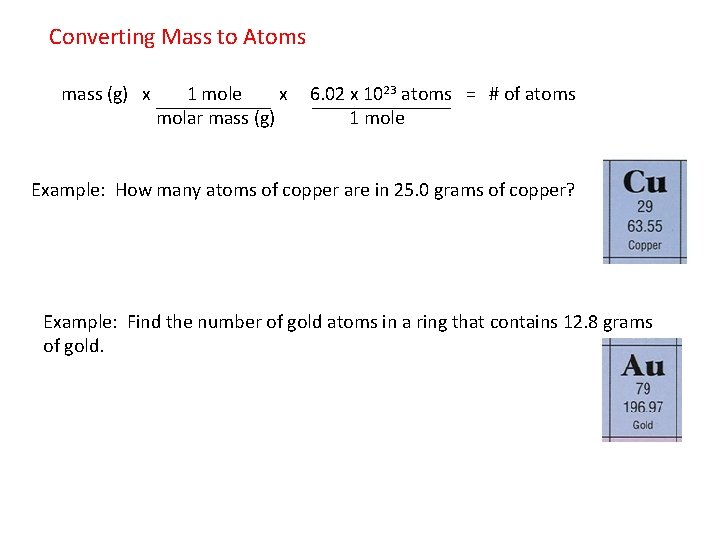 Source: slidetodoc.com
Source: slidetodoc.com
The molecular formula for Sodium Hydroxide is NaOH. How many atoms are in 15g of lead. The mass of one mole of lead Pb atoms is 2072 g. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. E none of the above.
 Source: youtube.com
Source: youtube.com
Turn on Show molar mass. Avogadros Number 1 mole 6022 1023particles and the relative atomic mass of copper 1 mole of copper 63546 g are the equalities required to make this conversion. Use this page to learn how to convert between moles Cu and gram. One mole is defined as the amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12 which is 602 10 23 particles. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
Multiply moles of Ca by the conversion factor molar mass of calcium 4008 g Ca 1 mol Ca which then allows the cancelation of moles leaving grams of Ca. 1 mole is equal to 1 moles CuSO4 or 1596086 grams. The total number of atoms in a substance can also be determined by using the relationship between grams moles and atoms. How do you find the moles of copper sulfate. Number of moles in 6 grams 6g x 1 mole 63546g.
 Source: pinterest.com
Source: pinterest.com
Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. The CuSO4 molecule is made up of six atoms one copper one sulfur and four oxygen. The molar mass of Cu 63546 grams per mole. Keeping this in consideration how do you calculate moles of CuSO4. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles Cu or 63546 grams. You can view more details on each measurement unit. Its easier to work with grams so convert the mass. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. Note that rounding errors may occur so always check the results.
 Source: youtube.com
Source: youtube.com
Molecular weight of CuSO4 5H2O or grams The SI base unit for amount of substance is the mole. How many atoms are in 100g of gold. The mass in grams of one mole of any substance elements or compounds. Its easier to work with grams so convert the mass. There fore since the number of atoms in 1 mole is 6022 x 1023 atoms per mole.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is one mole of cu by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






