Your How many grams is one mole of aluminum oxide images are available. How many grams is one mole of aluminum oxide are a topic that is being searched for and liked by netizens today. You can Get the How many grams is one mole of aluminum oxide files here. Download all free images.
If you’re searching for how many grams is one mole of aluminum oxide pictures information related to the how many grams is one mole of aluminum oxide interest, you have pay a visit to the ideal blog. Our website frequently provides you with suggestions for refferencing the highest quality video and picture content, please kindly hunt and locate more informative video articles and graphics that match your interests.
How Many Grams Is One Mole Of Aluminum Oxide. Finally convert the number of moles of aluminum to grams of aluminum by multiplying by its molar mass. Molecular weight of aluminum oxide or grams The molecular formulation for aluminum oxide is Al2O3. Number of aluminum ions per molecule 2. 408 grams of aluminum oxide is equal to about 4 moles or 24089 E24 molecules.
 Warmup 1 Calculate The Molar Mass Of Sbi3 502 46 G Mole 2 According To The Equation Below How Many Moles Of Aluminum Oxide Can Be Made From 6 8 Moles Ppt Download From slideplayer.com
Warmup 1 Calculate The Molar Mass Of Sbi3 502 46 G Mole 2 According To The Equation Below How Many Moles Of Aluminum Oxide Can Be Made From 6 8 Moles Ppt Download From slideplayer.com
1mol Al 270g Al. Number of aluminum ions per molecule 2. 4 moles27 gmole 108 g. Find mass of aluminum in the given mass of aluminum oxide. Hence 5 mol Al and 375 mol O 2 are needed 5. Finally convert back to grams of oxygen using the molar mass.
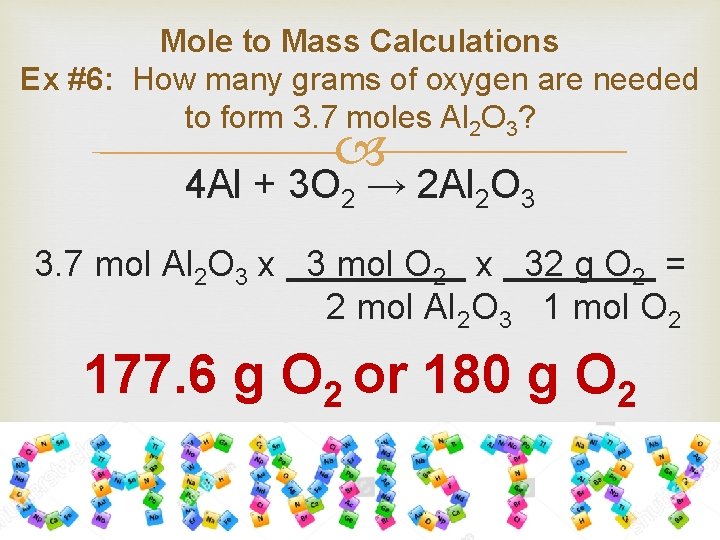
Calculate the number of grams of aluminum oxide produced.
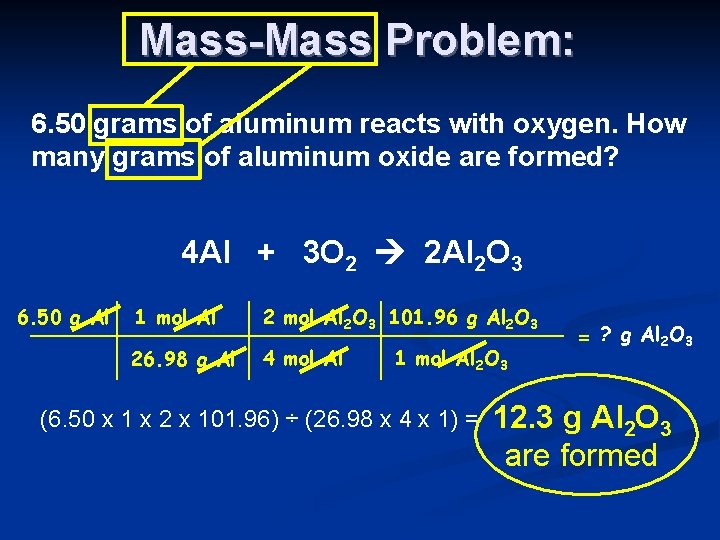
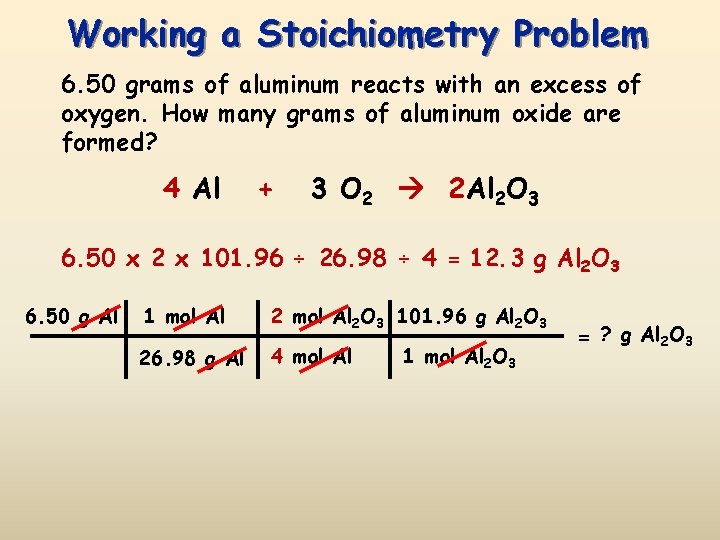
Finally convert the number of moles of aluminum to grams of aluminum by multiplying by its molar mass. Note that rounding errors may occur so always check the results. 40 moles Al 2 moles Al 2 O 3 4 moles Al There is enough oxygen to make 27 moles__ moles of aluminum oxide. Aluminum oxide is Al2O3. 2 grams aluminum 1 mole Al2698 grams6022 X 10231 moleAl 362. When aluminum is burned in excess oxygen aluminum oxide is produced.
 Source: youtube.com
Source: youtube.com
Molecular weight of Aluminium Oxide or grams The molecular formula for Aluminium Oxide is Al2O3. 3Find its molecular mass 2 23 3 16 units 46 48 94 units. 4Find mass of Aluminum in 1 mole of aluminum oxide 46 grams in 94 grams. Finally convert back to grams of oxygen using the molar mass. The SI base unit for amount of substance is the mole.
 Source: slidetodoc.com
Source: slidetodoc.com
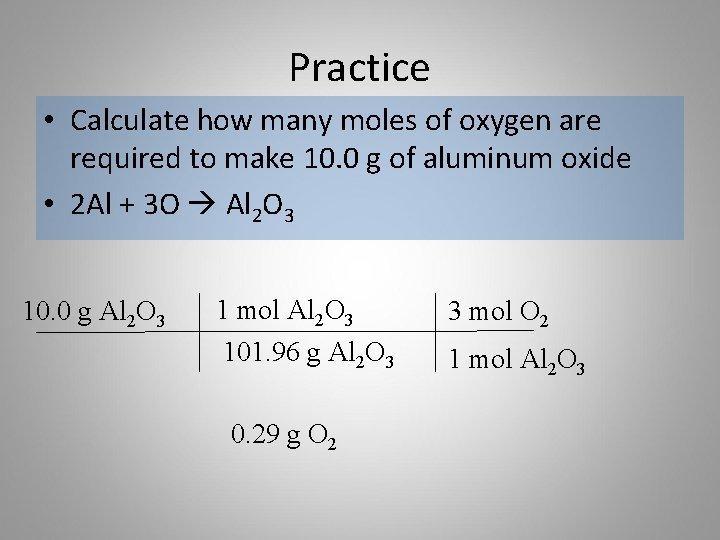
How many grams of ironIII chloride are produced when 153 g of iron react with excess chlorine gas. Mol-1 033 moles. According to the following reaction how many grams of sulfuric acid are necessary to form 0424 moles aluminum sulfate. How many moles of aluminum oxide should form. Hence 5 mol Al and 375 mol O 2 are needed 5.
 Source: slidetodoc.com
Source: slidetodoc.com
Number of aluminum ions per molecule 2. Add up the molar mass of each and every atom in a formula unit of the compound. 1 mol product 1 mol O 2 2 mol MgO 2 mol O 2. 2 How many grams of aluminum oxide are formed when 250 grams of Aluminum are reacted with oxygen gas. Mol-1 033 moles.
 Source: pinterest.com
Source: pinterest.com
How many atoms are in 10 grams of aluminum. The molar mass of aluminium is 27 grams per mole. Number of Al 2 O 3 molecules in 5 x 10-3 mole 6032 x 10 23x 5 x 10-3 30115 x 10 20. Therefore the mass of oxygen produced rounded to three significant figures is 225g. 2 How many grams of aluminum oxide are formed when 250 grams of Aluminum are reacted with oxygen gas.
 Source: pinterest.com
Source: pinterest.com
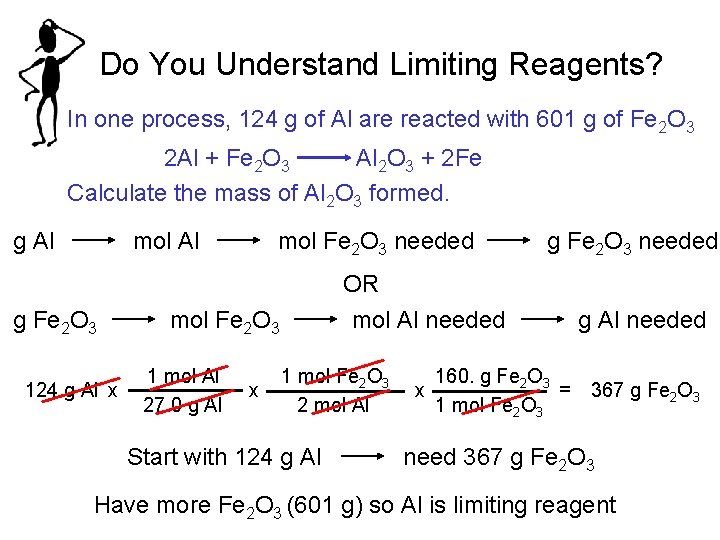
40 moles of aluminum are placed in a container with 40 moles of oxygen. 1 mole is the same as 1 moles aluminum oxide or 101961276 grams. Aluminum oxide has a molar mass of 101961 grams per mole. 1 mole is equal to 1. You can view more details on each measurement unit.
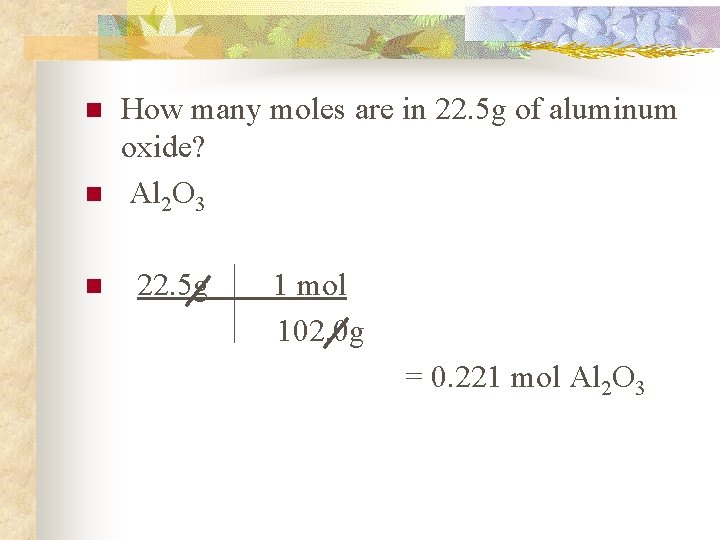 Source: slidetodoc.com
Source: slidetodoc.com
How many atoms are in 10 grams of aluminum. 23 16 3Find its molecular mass 2 23 3 16 units 46 48 94 units. 1020 gmol The molar mass of a compound is just the mass of one mole of the compound. 94 grams of aluminum oxide contains 46 grams of aluminum. 216 gmole 32 gmole.
 Source: slidetodoc.com
Source: slidetodoc.com
Structure of Aluminum Oxide. 2 mol Mg 2 mol MgO 1 mol Mg. Use this page to learn how to convert between moles aluminum oxide and gram. 408 grams of aluminum oxide is equal to about 4 moles or 24089 E24 molecules. 4 moles27 gmole 108 g.
 Source: slidetodoc.com
Source: slidetodoc.com
How many moles are in 1000 grams of eqAl_2O_3 eq. When one mole of aluminum reacts with excess oxygen how many moles of aluminum oxide will be produce View the full answer. How many grams of oxygen are required to produce 075 moles of Al 2O 3. How many moles are in 1000 grams of eqAl_2O_3 eq. 2 grams aluminum 1 mole Al2698 grams6022 X 10231 moleAl 362.
 Source: slidetodoc.com
Source: slidetodoc.com
Use this page to learn how to convert between moles aluminum oxide and gram. What percent of aluminum oxide is oxygen. 4 Al s 3 O2 g —– 2 Al2O3 s I. Then by stoichiometry of the reaction the following amounts of mass react and are produced. Calculate the number of grams of aluminum oxide produced.
 Source: slideplayer.com
Source: slideplayer.com
Find mass of aluminum in the given mass of aluminum oxide. X 1mol Al2O3 2mol Al X 1020g Al2O3 1mol Al2O3 472g Al2O3 Answer _____. Mol-1 033 moles. 1 mole is equal to 1. Add up the molar mass of each and every atom in a formula unit of the compound.
 Source: in.pinterest.com
Source: in.pinterest.com
1 mole is equal to 1 moles Aluminium Oxide or 101961276 grams. The molar mass of aluminium is 27 grams per mole. 227 gmole 316 gmole 102 gmole. Add up the molar mass of each and every atom in a formula unit of the compound. 2 mol Mg 2 mol MgO 1 mol Mg.
 Source: pinterest.com
Source: pinterest.com
500 g NaClO3 x 1 mole NaClO3 10644 g NaClO3 x 3 moles O2 2 moles NaClO3 x 31998 g O2 1 mole O2 2254 g of O2. Number of aluminum ions in mole Al 2 O 3 30115 x 10 20 x 2 6023 x 10 20. Assume oxygen has a molar mass of 16 gmol and aluminum has a molar mass of 27 gmol. 1 mole is equal to 1 moles aluminum oxide or 101961276 grams. So 1 mole of ALUMINIUM OXIDE will weigh 102 g 1 mole of any pure substance accommodates Avogrados variety of molecules thats 60231023.
 Source: pinterest.com
Source: pinterest.com
When aluminum is burned in excess oxygen aluminum oxide is produced. Molecular weight of Aluminium Oxide or grams. Find mass of aluminum in the given mass of aluminum oxide. How many grams of oxygen are required to produce 075 moles of Al 2O 3. 1 mole is equal to 1 moles aluminum oxide or 101961276 grams.
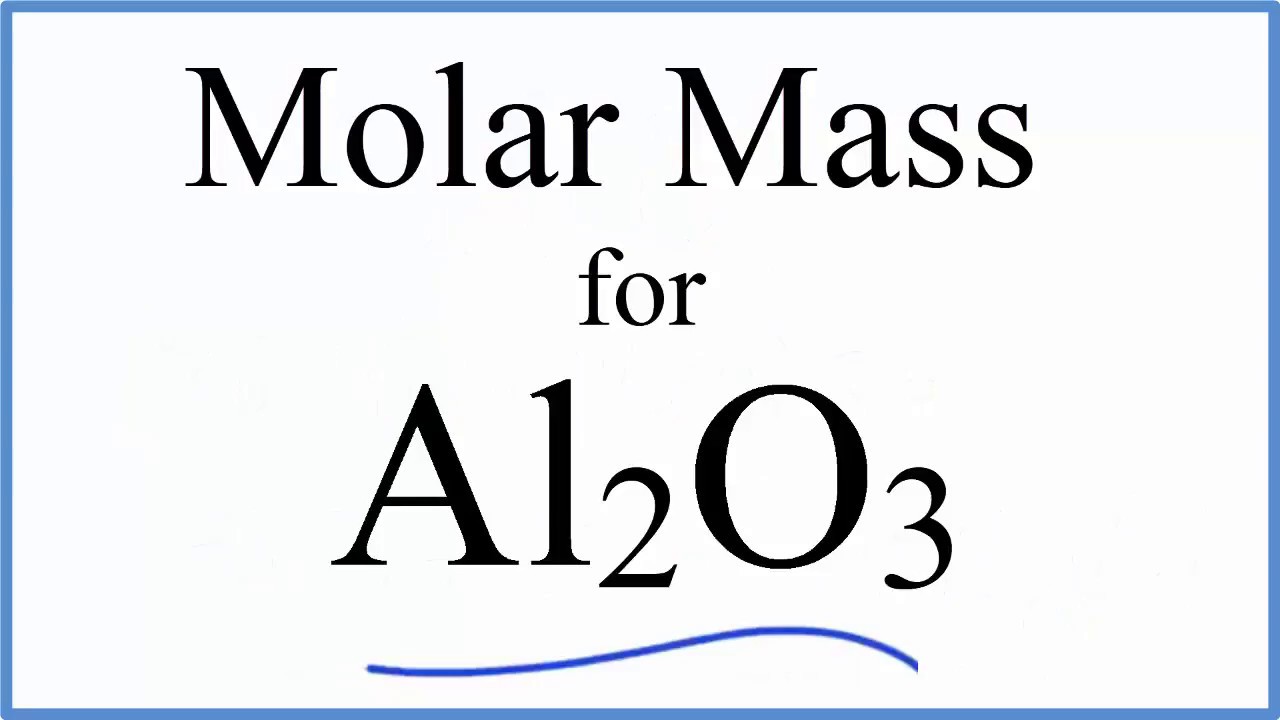 Source: youtube.com
Source: youtube.com
What percent of aluminum oxide is oxygen. Hence 5 mol Al and 375 mol O 2 are needed 5. 94 grams of aluminum oxide contains 46 grams of aluminum. How do you find atoms in aluminum. 2 mol Mg 2 mol MgO 1 mol Mg.
 Source: pinterest.com
Source: pinterest.com
Then by stoichiometry of the reaction the following amounts of mass react and are produced. When aluminum is burned in excess oxygen aluminum oxide is produced. The molar mass of aluminium is 27 grams per mole. Thus the total number of moles in 9 grams of aluminium 9g27g. Since there is 2 moles of Aluminium per mole of Al 2 O 3 you multiplly the number of moles calculated for aluminum oxide by 2.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is one mole of aluminum oxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.