Your How many grams is one mole of aluminum images are ready in this website. How many grams is one mole of aluminum are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams is one mole of aluminum files here. Find and Download all royalty-free photos and vectors.
If you’re looking for how many grams is one mole of aluminum pictures information connected with to the how many grams is one mole of aluminum keyword, you have visit the ideal site. Our website frequently gives you hints for seeking the maximum quality video and picture content, please kindly surf and find more enlightening video articles and graphics that fit your interests.
How Many Grams Is One Mole Of Aluminum. Be aware that rounding errors could happen so. Click to see full answer. 641 g 270 g mol1 237 mol. Use this page to learn how to convert between moles Aluminum and gram.
 Compute The Difference In Masses Of One Mole Each Of Aluminium Atoms And On Youtube From youtube.com
Compute The Difference In Masses Of One Mole Each Of Aluminium Atoms And On Youtube From youtube.com
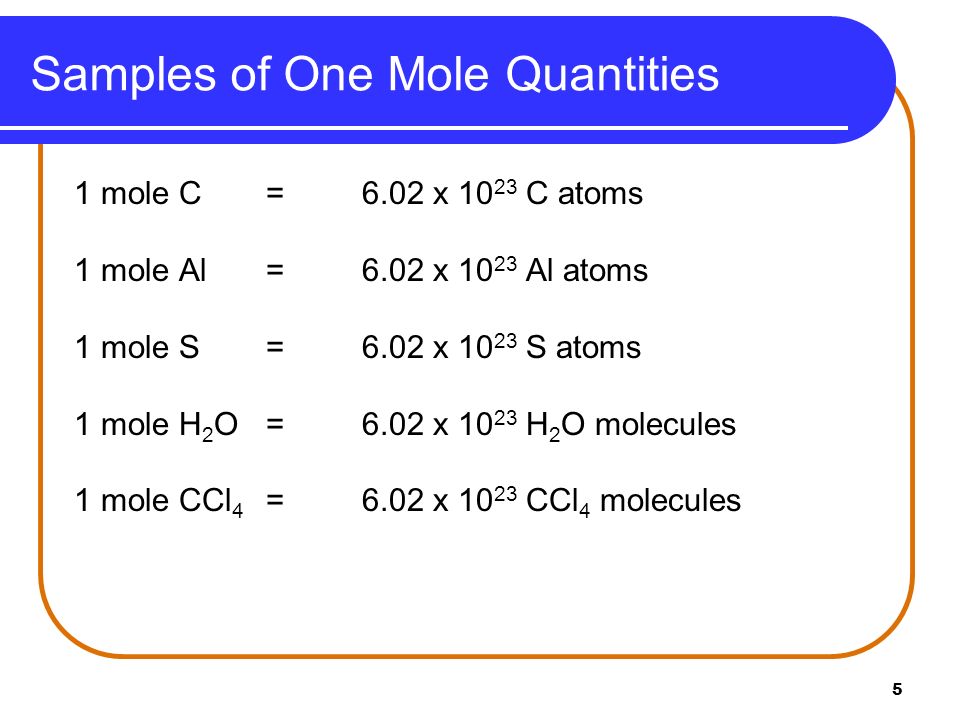
As you know one mole of any element contains exactly 60221023 atoms of that element. It also tells you how many grams of alum you have in one mole of alum add up the atomic weights. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5. 1 mole is equal to 1 moles Aluminum or 26981538 grams. What is the mass of 0250 moles of aluminum. The SI base unit for amount of substance is the mole.
And so we take the quotient.
Use this page to learn how to convert between grams Aluminum and mole. The SI base unit for quantity of substance is the mole. Thus the total number of moles in 9 grams of aluminium 9g27g. Ok this question is not complete. Use this page to learn how to convert between moles Aluminium Bromide and gram. 1 mole is equal to 1 moles Aluminium Bromide or 266693538 grams.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between moles Aluminum and gram. Dividing 269860210 23 yields a mass of 44810 23 g for one aluminum atom. Use this page to learn how to convert between grams Aluminum and mole. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5. The SI base unit for amount of substance is the mole.
 Source: studylib.net
Source: studylib.net
As you know one mole of any element contains exactly 60221023 atoms of that element. Connect the information given with what youre trying to find. Dividing 269860210 23 yields a mass of 44810 23 g for one aluminum atom. Use this page to learn how to convert between moles Aluminium Bromide and gram. Aluminium Chloride molecular weight.
 Source: nist.gov
Source: nist.gov
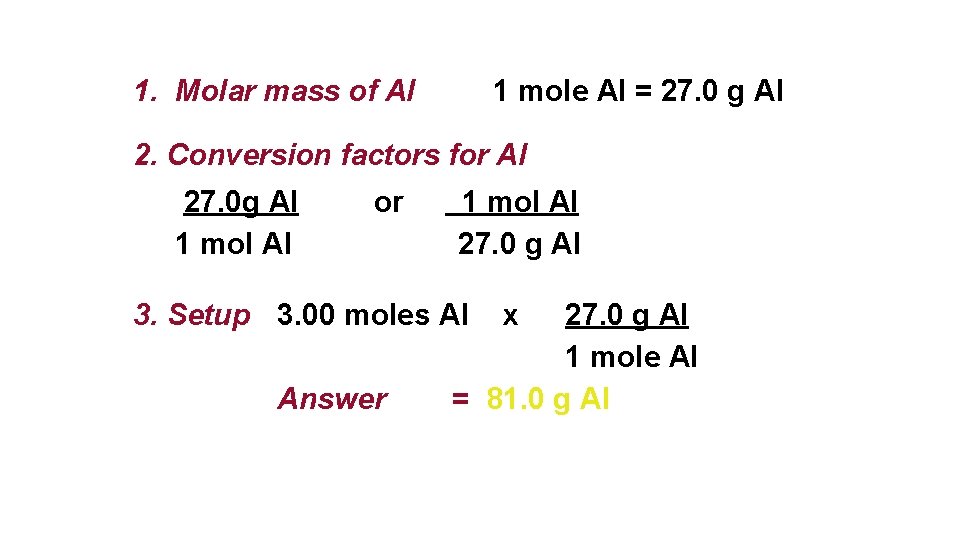
What is the mass of 430 moles of aluminum. Therefore 1 mole of aluminium will weigh 27 grams. 03032016 33 grams aluminum 1 mole Al2698 grams 12 moles of aluminum What number of moles aluminum exist in 1000 grams of aluminum. The formula of alum tells you that one mole of alum contains one mole of Al. 7500 mols of Al.
 Source: slideplayer.com
Source: slideplayer.com
7500 mols of Al. Thus the total number of moles in 9 grams of aluminium 9g27g. Therefore 1 mole of aluminium will weigh 27 grams. Ok this question is not complete. Therefore 1 mole of aluminium will weigh 27 grams.
 Source: texasgateway.org
Source: texasgateway.org
Subsequently theres 74996 mols of Al. As you know one mole of any element contains exactly 60221023 atoms of that element. 1 mole is equal to 1 moles Aluminium Bromide or 266693538 grams. Thus the total number of moles in 9 grams of aluminium 9g27g. 641 g 270 g mol1 237 mol.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
How many aluminum atoms are present in this molar quantity. 1 mole is equal to 1 moles Aluminum or 26981538 grams. Dividing 269860210 23 yields a mass of 44810 23 g for one aluminum atom. Convert 9500g of iron to number of atoms in the sample. Ok this question is not complete.
 Source: youtube.com
Source: youtube.com
You can view more details on each measurement unit. Connect the information given with what youre trying to find. As you know one mole of any element contains exactly 60221023 atoms of that element. The unit used here is the unified atomic mass unit u. 641 g 270 g mol1 237 mol.
 Source: sciencephoto.com
Source: sciencephoto.com
1 mole of aluminium 27g 60231023 noof atoms. The formula of alum tells you that one mole of alum contains one mole of Al. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5. Convert 9500g of iron to number of atoms in the sample. 1 mole of Aluminum is equal to 26981538 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
And so we take the quotient. You can view more details on each measurement unit. The SI base unit for quantity of substance is the mole. Subsequently theres 74996 mols of Al. 03032016 33 grams aluminum 1 mole Al2698 grams 12 moles of aluminum What number of moles aluminum exist in 1000 grams of aluminum.
 Source: youtube.com
Source: youtube.com
So 1 mole of ALUMINIUM OXIDE will weigh 102 g 1 mole of any pure substance accommodates Avogrados variety of molecules thats 60231023. 0250 moles 270g 675 g Al 1 mole 7. Therefore 1 mole of aluminium will weigh 27 grams. Dividing 269860210 23 yields a mass of 44810 23 g for one aluminum atom. 641 g 270 g mol1 237 mol.
 Source: sciencephoto.com
Source: sciencephoto.com
1 mole is equal to 1 moles Aluminium or 26981538 grams. Thus the total number of moles in 9 grams of aluminium 9g27g. So 1 mole of ALUMINIUM OXIDE will weigh 102 g 1 mole of any pure substance accommodates Avogrados variety of molecules thats 60231023. 35 Related Question Answers Found. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
How many grams is equal to 348 x 1022 atoms of tin. What is the mass of 430 moles of aluminum. The formula of alum tells you that one mole of alum contains one mole of Al. 1 mole is the same as 1 moles aluminum oxide or 101961276 grams. 27g of aluminium contains 60231023 noof atoms.
 Source: slidetodoc.com
Source: slidetodoc.com
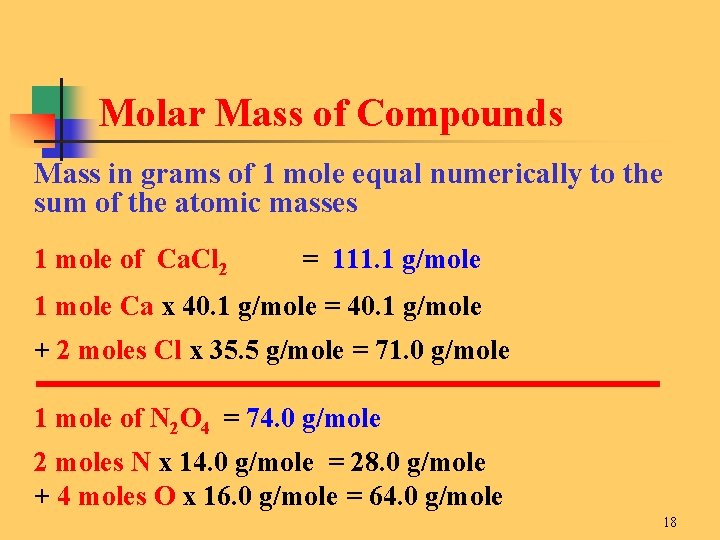
Molecular weight of Aluminium or grams The molecular formula for Aluminium is Al. Therefore 1 mole of aluminium will weigh 27 grams. Type in your own numbers in the form to convert the units. Molecular weight of Aluminium or grams The molecular formula for Aluminium is Al. You would need to have a mass of aluminum to find the number of moles.
 Source: m.youtube.com
Source: m.youtube.com
Subsequently theres 74996 mols of Al. The formula of alum tells you that one mole of alum contains one mole of Al. Therefore 1 mole of aluminium will weigh 27 grams. 9500 g Fe 1 mole 602 x 1023 atoms 10 x 1026 atoms 558g 1 mole 6. Now the moles of aluminum in its given mass is determined as shown below Number of.
 Source: studylib.net
Source: studylib.net
Be aware that rounding errors could happen so. 1 grams Aluminum is equal to 0037062379468509 mole. 1 mole is the same as 1 moles aluminum oxide or 101961276 grams. This compound is also called Aluminium Chloride. 1 mole of aluminium 27g 60231023 noof atoms.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is one mole of aluminum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






