Your How many grams is one mole of al images are available in this site. How many grams is one mole of al are a topic that is being searched for and liked by netizens today. You can Get the How many grams is one mole of al files here. Find and Download all free photos.
If you’re searching for how many grams is one mole of al pictures information linked to the how many grams is one mole of al interest, you have come to the ideal site. Our site always provides you with suggestions for downloading the highest quality video and picture content, please kindly search and locate more enlightening video content and images that match your interests.
How Many Grams Is One Mole Of Al. This means that the mass of one mole of aluminium atoms will be 26982 g. The SI base unit for amount of substance is the mole. Use this page to learn how to convert between moles AlCl and gram. You can view more details on each measurement unit.
 Converting Grams To Moles Grams To Moles Mass To Moles Mole Conversion From pinterest.com
Converting Grams To Moles Grams To Moles Mass To Moles Mole Conversion From pinterest.com
The molar mass of Aluminium is approximately equal to 2698gmol ie in 2698grams of aluminium there is one mole of aluminium atoms one mole is equal to Avogadros constant 6022140761023 So to find the amount of moles of Al you have you can simply divide 135 by 2698 5 mol. You can view more details on each measurement unit. You can view more details on each measurement unit. 1 mole is equal to 1 moles AlCl or 62434538 grams. 1 Given equation is. Multiply by one mole for units to cancel540 grams Al.
Notice that aluminium is said to have an atomic mass of 26982 u.
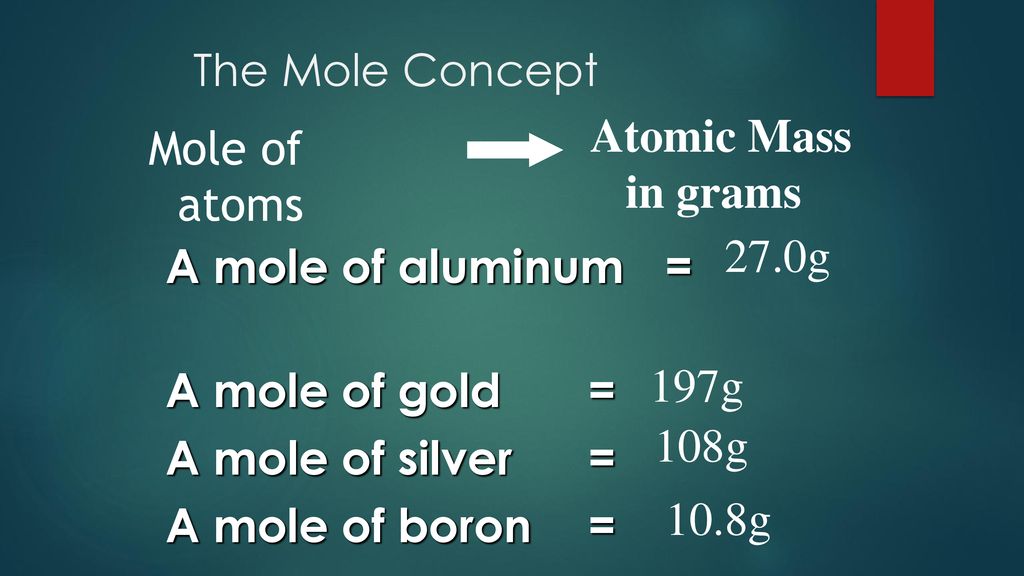
1 mole is equal to 1 moles Al or 26981538 grams. 1 Given equation is. 1 mole is equal to 1 moles Al OH3 or 78003558 grams. This means that the mass of one mole of aluminium atoms will be 26982 g. Dividing 26986021023 yields a mass of 4481023 g for one aluminum atom. 1 mole is equal to 1 moles Aluminium or 26981538 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
The mass in grams of a mole of sulfur sulfurs molar mass can be found on a periodic table of the elements to be 32 grams per mole. You can view more details on each measurement unit. How many grams of Al0 can form from 318 g of Al. For this you need the atomic mass of Al. Chemistry questions and answers.
 Source: slideplayer.com
Source: slideplayer.com
How many kg of Titanium III chloride was produced from 52 kg. How many moles of aluminum oxide are produced according to the reaction below given that you start with 100 grams of Al and 190 grams of O2. Each formula unit of aluminum sulfate has three sulfur atoms so your 3 moles of aluminum sulfate will contain 9 moles of sulfur atoms. You may ask how is it possible for ever substances. Weights of atoms and isotopes are from NIST article.
 Source: slidetodoc.com
Source: slidetodoc.com
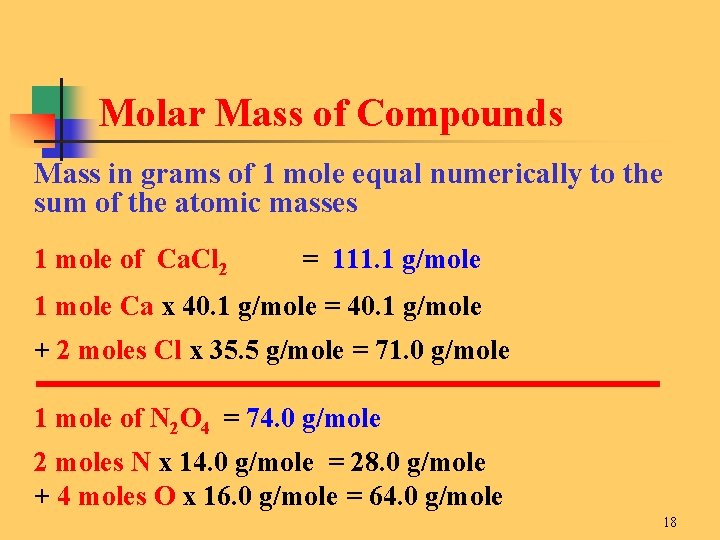
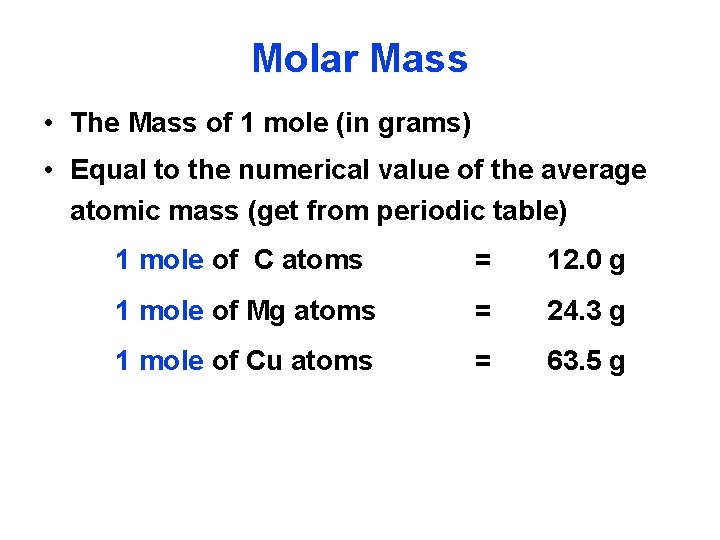
One mole of Al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. Molecular weight of AlCl or grams. 1 mole is equal to 1 moles Al or 26981538 grams. The unit used here is the unified atomic mass unit u. One mole of aluminum atoms has a mass of 2698 grams and contains 6021023 atoms.
 Source: slideplayer.com
Source: slideplayer.com
One mole of Al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. The unit used here is the unified atomic mass unit u. The answer is 26981538. 1 u is equal to 112 the mass of one atom of carbon-12 Molar mass molar weight is the mass of one mole of a substance and is expressed in gmol. AlOH 3 780 gmole 1 mole Al 270 gmole 3 mol O 160 gmole 3 mol H 101 gmole 270 g 480 g 303 g 780 g Solution.
 Source: in.pinterest.com
Source: in.pinterest.com
Multiply by one mole for units to cancel540 grams Al. Dividing 26986021023 yields a mass of 4481023 g for one aluminum atom. Use this page to learn how to convert between grams Al OH3 and mole. 1 mole is equal to 1 moles Al OH3 or 78003558 grams. Note that rounding errors may occur so always check the results.
 Source: hu.pinterest.com
Source: hu.pinterest.com
The SI base unit for amount of substance is the mole. As you know one mole of any element contains exactly 60221023 atoms of that element. Probably the 10 grams aluminum because the mass of aluminum is lighter than irons mass so more moles of aluminum. We assume you are converting between grams Al and mole. The answer is 26981538.
 Source: pinterest.com
Source: pinterest.com
1mol Al 270g Al. Type in your own numbers in the form to convert the units. Weights of atoms and isotopes are from NIST article. Multiply by one mole for units to cancel540 grams Al. This means the amount of atoms would be equal to.
 Source: pinterest.com
Source: pinterest.com
Probably the 10 grams aluminum because the mass of aluminum is lighter than irons mass so more moles of aluminum. Probably the 10 grams aluminum because the mass of aluminum is lighter than irons mass so more moles of aluminum. 4 Al s 302 g 2 A103 8 Step 1. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. 1 Dozen copy12 cpoies.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles Al OH3 and gram. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Add up the molar mass of each and every atom in a formula unit of the compound. 1 mole is equal to 1 moles AlCl or 62434538 grams. The unit used here is the unified atomic mass unit u.
 Source: pinterest.com
Source: pinterest.com
4 Ti — 4 TiCl. How many moles of aluminum oxide are produced according to the reaction below given that you start with 100 grams of Al and 190 grams of O2. You may ask how is it possible for ever substances. How many kg of Titanium III chloride was produced from 52 kg. 4 Ti — 4 TiCl.
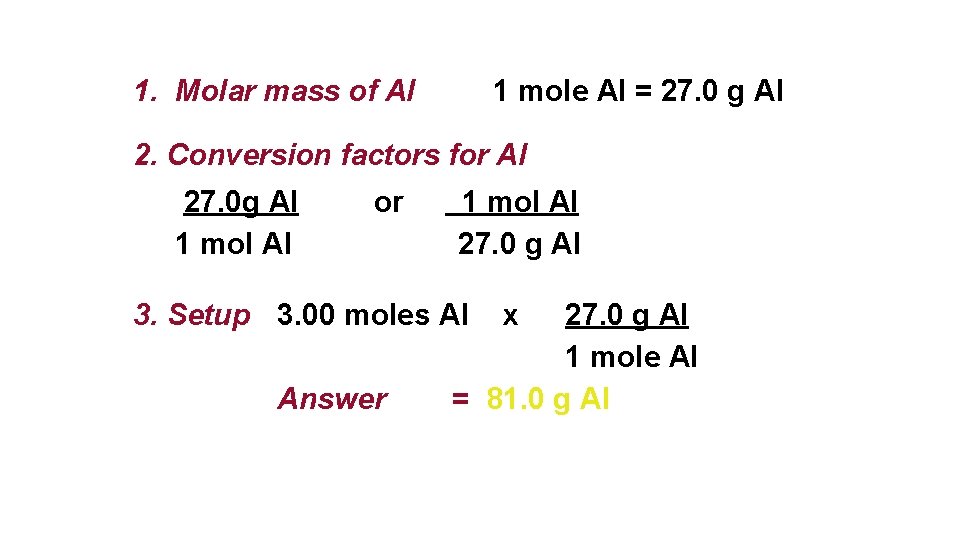 Source: slidetodoc.com
Source: slidetodoc.com
Probably the 10 grams aluminum because the mass of aluminum is lighter than irons mass so more moles of aluminum. Show the strategy for solving this problem. X 1mol Al2O3 2mol Al X 1020g Al2O3 1mol Al2O3 472g Al2O3 Answer _____ 3 A sample of TiCl4 is reacted with Titanium metal to produce Titanium III chloride. One mole of aluminum atoms has a mass of 2698 grams and contains 6021023 atoms. You can view more details on each measurement unit.
 Source: slideplayer.com
Source: slideplayer.com
How many kg of Titanium III chloride was produced from 52 kg. Weights of atoms and isotopes are from NIST article. You can view more details on each measurement unit. Above all is basics here comes advanced one One mole of any substance Gram molecular weight or gram atomic weight of that susbstance. You can view more details on each measurement unit.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. Type in your own numbers in the form to convert the units. Show the conversions required to solve this problem and calculate. 250g Al X. You may ask how is it possible for ever substances.
 Source: es.pinterest.com
Source: es.pinterest.com
One mole of Al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. Use this page to learn how to convert between moles Al OH3 and gram. 250g Al X. Show the conversions required to solve this problem and calculate. As you know one mole of any element contains exactly 60221023 atoms of that element.
 Source: gr.pinterest.com
Source: gr.pinterest.com
How many grams of Al0 can form from 318 g of Al. 1 u is equal to 112 the mass of one atom of carbon-12 Molar mass molar weight is the mass of one mole of a substance and is expressed in gmol. Note that rounding errors may occur so always check the results. How many kg of Titanium III chloride was produced from 52 kg. 4 Al s 302 g 2 A103 8 Step 1.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is one mole of al by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.