Your How many grams is one mole of ag images are available in this site. How many grams is one mole of ag are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams is one mole of ag files here. Find and Download all royalty-free vectors.
If you’re looking for how many grams is one mole of ag images information related to the how many grams is one mole of ag topic, you have pay a visit to the ideal site. Our site always gives you suggestions for seeing the highest quality video and image content, please kindly surf and find more enlightening video content and graphics that match your interests.
How Many Grams Is One Mole Of Ag. 1 grams Ag is equal to 00092705727916105 mole. Now we have to perform moles to grams calculation. Use this page to learn how to convert between grams AgNO3 and mole. N 5988 g 18015 gmol 3324 mol.

Type in your own numbers in the form to convert the units. The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results. Use this page to learn how to convert between grams AgBr and mole. 0745 1023 mol C. Molar mass of Ag2O 210816g 216g 232gmole 232g of Ag2O contains 216g of Ag Therefore395g216395232g of Ag 3678g of Ag Again 108g of Ag 1 mole Therefore3678 3678108 034 mole.
1 mole is equal to 1 moles Ag or 1078682 grams.
M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. Molecular weight of Silver or grams. Note that rounding errors may occur so always check the results. TextMolar Mass 107868 for calculations tap Molar Mass Calculator By using moles to grams formula. Note that rounding errors may occur so always check the results. Type in your own numbers in the form to convert the units.

Type in your own numbers in the form to convert the units. 1 mole of ag 1079 g of Ag 1079 g Ag 1 mole Ag and 1 mole Ag 1079 g Ag 750 mole Ag X 1079 Ag 1 mole Ag 809 g of Ag. The amount of a substance that contains the same number of entities as there are atoms in 12 g of carbon-12. How many grams are in once. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
What about 927 x10 -3 moles. The SI base unit for amount of substance is the mole. 0745 1023 mol C. 1 mole is equal to 1 moles Silver or 1078682 grams. 1 mole of ag 1079 g of Ag 1079 g Ag 1 mole Ag and 1 mole Ag 1079 g Ag 750 mole Ag X 1079 Ag 1 mole Ag 809 g of Ag.
 Source: toppr.com
Source: toppr.com
Note that rounding errors may occur so always check the results. Note that rounding errors may occur so always check the results. Type in your own numbers in the form to convert the units. 1 mole is equal to 1 moles Silver or 1078682 grams. Note that rounding errors may occur so always check the results.
 Source: mammothmemory.net
Source: mammothmemory.net
1 The mole or mol represents a certain number of objects. Molar mass of Ag2O 210816g 216g 232gmole 232g of Ag2O contains 216g of Ag Therefore395g216395232g of Ag 3678g of Ag Again 108g of Ag 1 mole Therefore3678 3678108 034 mole. Determine the mass in grams of. 1 The mole or mol represents a certain number of objects. Note that rounding errors may occur so always check the results.

Similar chemical formulas. Calculate the moles of Ag massmolar mass. 0745 1024 mol B. How many grams are in once. Type in your own numbers in the form to convert the units.
 Source: slideplayer.com
Source: slideplayer.com
1 mole is equal to 1 moles Ag or 1078682 grams. What about 927 x10 -3 moles. The molecular formula for Silver is Ag. 1 mole is equal to 1 moles Ag2S or 2478014 grams. What is the mass of 225 x 1025 atoms of lead.
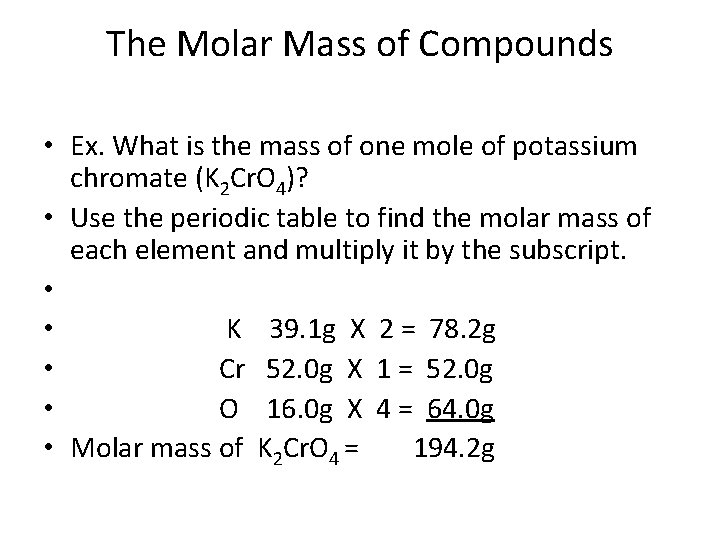 Source: slidetodoc.com
Source: slidetodoc.com
Teaspoons and grams for salt Teaspoons to grams Teaspoons to grams 2 teaspoons 118g 7 teaspoons 414g 3 teaspoons 178g 8 teaspoons 474g 4 teaspoons 237g 9 teaspoons 533g 5 teaspoons 296g 10 teaspoons 592g. Note that rounding errors may occur so always check the results. How many moles of Ag are in 451 grams of Ag2O. The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results.
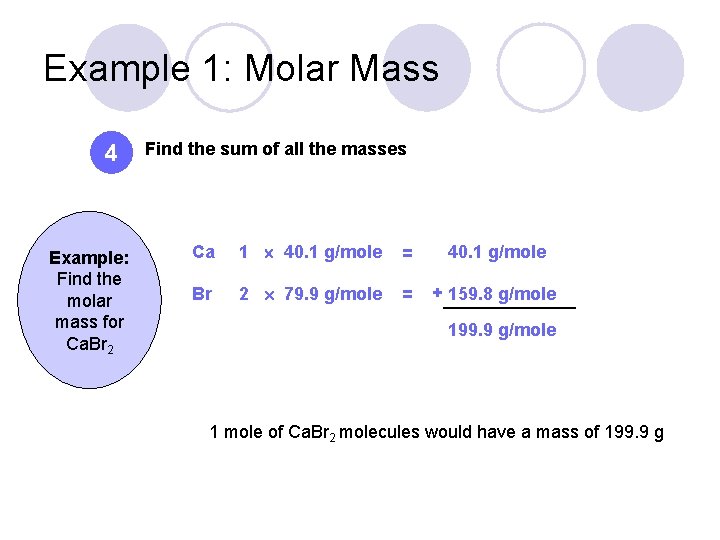 Source: slidetodoc.com
Source: slidetodoc.com
Molecular weight of Ag or mol The SI base unit for amount of substance is the mole. 0745 1023 mol C. Note that rounding errors may occur so always check the results. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Silver or 1078682 grams.
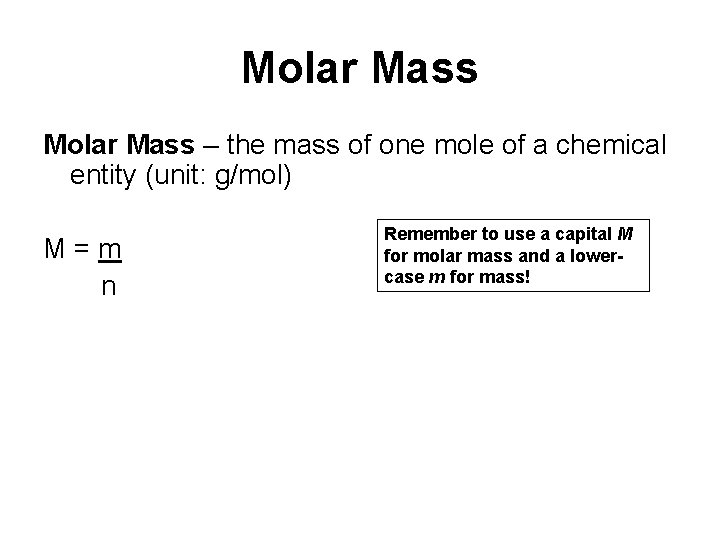 Source: slidetodoc.com
Source: slidetodoc.com
How many moles of Ag are in 451 grams of Ag2O. M n M m 3899 107868 m 420577332 g You can also verify the results by fetching the values in our free moles to grams calculator. Use this page to learn how to convert between moles Ag2S and gram. 2 AgNO 3 H 2S Ag 2S 2 HNO 3 1000 g Ag 2S 0403 mol Ag 2S Step 1. 1g 10787 gmol 927 x10 -3 moles.
 Source: studylib.net
Source: studylib.net
Use this page to learn how to convert between grams AgBr and mole. 1 grams Ag is equal to 00092705727916105 mole. How many grams are in 150 moles of KMnO4. Convert the amount of known substance Ag 2S from grams to moles 11 How many moles of silver nitrate AgNO. Use this page to learn how to convert between grams AgBr and mole.
 Source: slideplayer.com
Source: slideplayer.com
The mole lab chemistry 1 acc answer key. Darmaidayxx and 3 more users found this answer helpful. 1137 g 301 g 0033 g 447 g. The SI base unit for amount of substance is the mole. Exactly 12 g of carbon-12 contains 6022 x 10 23 atoms.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Use this page to learn how to convert between moles Ag and gram. By use of Avogadro law constant 1 mole 602 x1023 atoms. 1 grams Ag is equal to 00092705727916105 mole. The SI base unit for amount of substance is the mole. Use this page to learn how to convert between grams AgBr and mole.
 Source: studylib.net
Source: studylib.net
Determine the mass in grams of. You can view more details on each measurement unit. Calculate the moles of Ag massmolar mass. Note that rounding errors may occur so always check the results. How many moles are in 25 grams of KMnO4.
 Source: slideplayer.com
Source: slideplayer.com
N 5988 g 18015 gmol 3324 mol. Note that rounding errors may occur so always check the results. Exactly 12 g of carbon-12 contains 6022 x 10 23 atoms. How many grams is 10 teaspoons. What about 927 x10 -3 moles.
 Source: clutchprep.com
Source: clutchprep.com
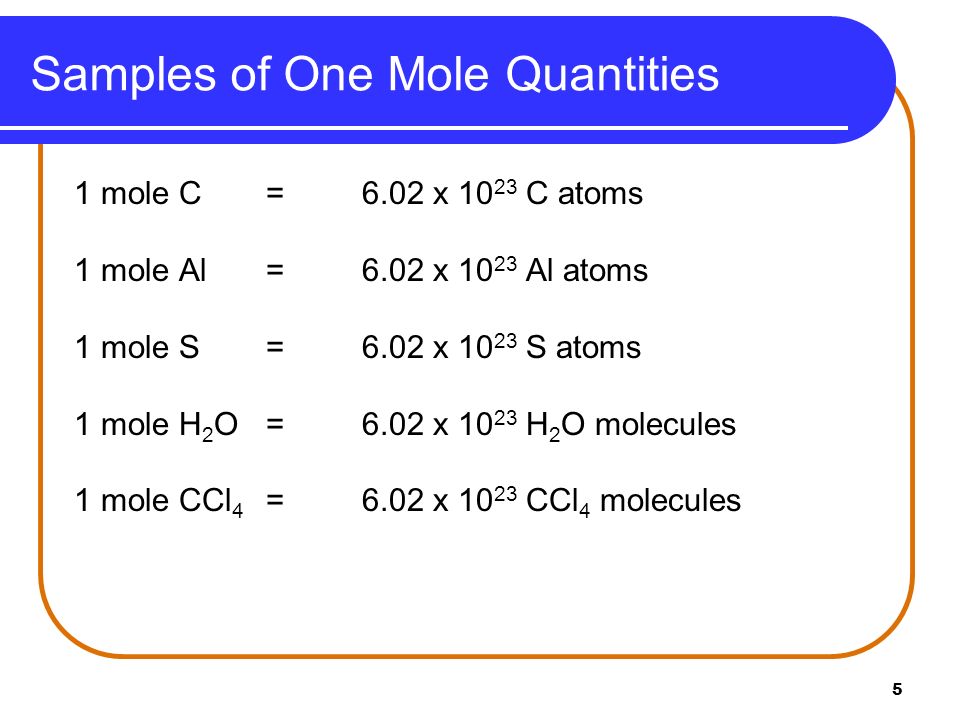
Use this page to learn how to convert between moles Ag2S and gram. Exactly 12 g of carbon-12 contains 6022 x 10 23 atoms. One mole of H 2 O molecules contains 6022 x 10 23 molecules. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. The SI base unit for amount of substance is the mole.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is one mole of ag by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






