Your How many grams is in 1 mole of sodium chloride images are ready in this website. How many grams is in 1 mole of sodium chloride are a topic that is being searched for and liked by netizens now. You can Download the How many grams is in 1 mole of sodium chloride files here. Find and Download all free vectors.
If you’re looking for how many grams is in 1 mole of sodium chloride images information connected with to the how many grams is in 1 mole of sodium chloride interest, you have visit the right blog. Our website always gives you suggestions for refferencing the maximum quality video and picture content, please kindly hunt and locate more informative video content and images that fit your interests.
How Many Grams Is In 1 Mole Of Sodium Chloride. 5 moles aluminum chloride to grams 66670269 grams. 1 mole of molecules is 6021023 molecules. The answer is 5844277. Type in your own numbers in the form to convert the units.
 Calculate The Mass Of 1 Mole Of Each One Of The Following A Nacl B Caco 3 C Feso Youtube From youtube.com
Calculate The Mass Of 1 Mole Of Each One Of The Following A Nacl B Caco 3 C Feso Youtube From youtube.com
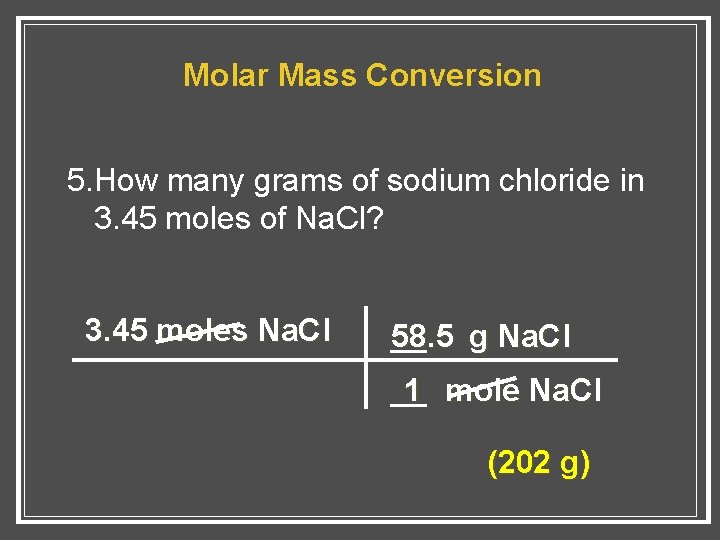
Use this page to learn how to convert between moles Sodium Chloride and gram. So we can calculate the mass by simply multiplying the number of moles and molar mass. Molecular weight of NaCl or grams This compound is also known as Sodium Chloride. Again 1 molecule of sodium chloride consists of 1 sodium atom. In order to work this out multiply Avogadros number - the one I stated at the start of this explanation - by 2. Mass 585 x 009 5265 g.
Again 1 molecule of sodium chloride consists of 1 sodium atom.
6 moles aluminum chloride to grams 80004323 grams. Not at all Slightly Kinda Very much Completely Still have questions. Hence 73125 grams of sodium chloride is present in 250mL. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. Note that rounding errors may occur so always check the results. This means that 1 MOLE of hydrogen atoms will weigh 1008 grams.
 Source: youtube.com
Source: youtube.com
Hence 73125 grams of sodium chloride is present in 250mL. We calculate this from the atomic masses of sodium and chlorine. Mass 140 moles 5844 g. Not at all Slightly Kinda Very much Completely Still have questions. 1 mole sodium chloride 585g 60231023 noof molecules.

This compound is also known as Magnesium Chloride. The molar mass of sodium Na is 2299 gmole and the molar mass of chlorine Cl is 3545 gmole I got these values from a periodic table so the molar mass of sodium chloride NaCl is 2299 3545 5844 gmole. One mole of sodium chloride has a mass of 585 g. In other words 1 mole of atoms is 6021023 atoms. 1 mole is equal to 1 moles Calcium Chloride or 110984 grams.
 Source: youtube.com
Source: youtube.com
Use this page to learn how to convert between moles NaCl and gram. At 20C 68F or 29315K at standard atmospheric pressure. Use this page to learn how to convert between moles Sodium Chloride and gram. The SI base unit for amount of substance is the mole. We calculate this from the atomic masses of sodium and chlorine.

This means that 1 MOLE of hydrogen atoms will weigh 1008 grams. Now you can see from the equation above that in 585g of NaCl there are 23g of sodium. There are 5265 g of sodium chloride in 100 mL of 09 M solution. You have 12 grams which equals 12585 021 moles. 2 moles aluminum chloride to grams 26668108 grams.

040 moles NaCl 5844 grams1 mole NaCl 23 grams sodium chloride How many moles of sodium chloride are in 400 mL of. Number of moles 09 x 01 009 mol. How many moles and grams of NaCl are present in 250 ml. Given volume of solution is 250ml or 025 liter. One mole of sodium chloride has a mass of 585 g.
 Source: pinterest.com
Source: pinterest.com
Barnuts barnuts The molar mass of NaCl is 5844 g mol. Given volume of solution is 250ml or 025 liter. 2 moles aluminum chloride to grams 26668108 grams. How many moles in 100 grams of sodium chloride. To work out the number of moles in 100mL of 09M solution.
 Source: unitshub.com
Source: unitshub.com
Note that rounding errors may occur so always check the results. 1 moles aluminum chloride to grams 13334054 grams. This compound is also known as Magnesium Chloride. 1 See answer Advertisement Advertisement ambientwaves is waiting for your help. Note that rounding errors may occur so always check the results.
 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
1 moles aluminum chloride to grams 13334054 grams. You have 12 grams which equals 12585 021 moles. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. Thereforethe noof atoms of sodium in sodium chloride 60231023 noof Na atoms. 1 mole is equal to 1 moles Sodium Chloride or 5844277 grams.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. So we can calculate the mass by simply multiplying the number of moles and molar mass. Note that rounding errors may occur so always check the results. This means that 1 MOLE of hydrogen atoms will weigh 1008 grams. 5 moles aluminum chloride to grams 66670269 grams.
 Source: meritnation.com
Source: meritnation.com
Use this page to learn how to convert between moles Sodium Chloride and gram. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. Note that rounding errors may occur so always check the results. Number of moles 09 x 01 009 mol. Note that rounding errors may occur so always check the results.
 Source: youtube.com
Source: youtube.com
How many moles and grams of NaCl are present in 250 ml. The answer is 5844277. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram. 6 moles aluminum chloride to grams 80004323 grams. 1 mole is equal to 1 moles NaCl or 5844277 grams.
 Source: itprospt.com
Source: itprospt.com
1 mole is equal to 1 moles Calcium Chloride or 110984 grams. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. Number of moles mol concentration M x volume L 100 mL 01 L.

Note that rounding errors may occur so always check the results. This will give you the number of molecules in 2 moles of a substance. Type in your own numbers in the form to convert the units. At 20C 68F or 29315K at standard atmospheric pressure. 1 moles aluminum chloride to grams 13334054 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
Use this page to learn how to convert between moles NaCl and gram. 1 See answer Advertisement Advertisement ambientwaves is waiting for your help. 5 moles aluminum chloride to grams 66670269 grams. Use this page to learn how to convert between moles Sodium Chloride and gram. 2 moles aluminum chloride to grams 26668108 grams.
 Source: youtube.com
Source: youtube.com
How many moles and grams of NaCl are present in 250 ml. How many grams are in 140 moles of sodium chloride NaCl. 6 moles aluminum chloride to grams 80004323 grams. At 20C 68F or 29315K at standard atmospheric pressure. 1 mole is equal to 1 moles Sodium Chloride or 5844277 grams.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is in 1 mole of sodium chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






