Your How many grams is 1 mole of naoh images are available in this site. How many grams is 1 mole of naoh are a topic that is being searched for and liked by netizens today. You can Get the How many grams is 1 mole of naoh files here. Find and Download all free vectors.
If you’re looking for how many grams is 1 mole of naoh images information linked to the how many grams is 1 mole of naoh interest, you have visit the right site. Our website frequently provides you with suggestions for seeing the highest quality video and picture content, please kindly search and find more informative video content and images that match your interests.
How Many Grams Is 1 Mole Of Naoh. 160 g S8 x 1 mol S8 x 8 mol Fe x 5585 g Fe 27. From the balanced equation above 2 moles of Na reacted to produce 80g of NaOH. The answer is 3999711. Na - 230 - 16 and H - 1 so the molecular weight is 23 16 I 40Thus 40 grams of NaOH equals one mole of NaOH and a 1 molar solution of NaOH will contain 40 grams of NaOH chemical.
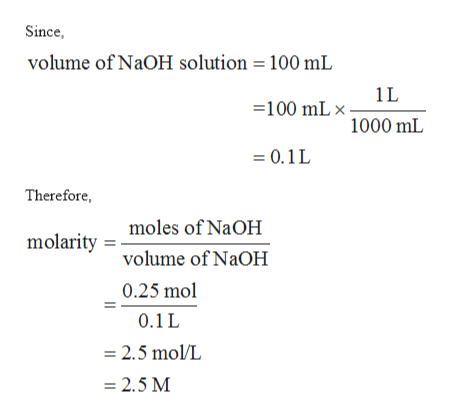 Answered What Is The Molarity M Of A N 10 Bartleby From bartleby.com
Answered What Is The Molarity M Of A N 10 Bartleby From bartleby.com
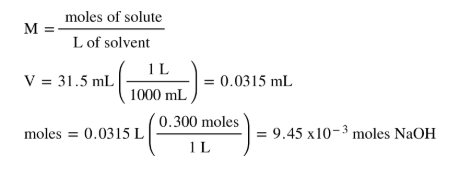
How many moles are in 1 gram. Therefore Xmol of Na will react to produce 40g of NaOH ie. The weight of one mole is 84 grams. Since sodium hydroxide reacts 11 with the KHP acid this also the number of moles of KHP needed for a complete reaction and neutralization. For this you need the atomic molecular mass of NaOH. 5 M N a O H is diluted to 8 0 0 ml then find molarity of resultant solution.
For this you need the atomic molecular mass of NaOH.
So to prepare 1N solution of NaOH we need to dissolve 40 gms of NaOH in 1000 ml of distilled water. 1 grams NaOH is equal to 0025001806380511 mole. Molarity Moles of solute Volume of solution in litres 200 M n 200 L. The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer. You can view more details on each measurement unit. 2g NaOH is needed for 1000ml solution.
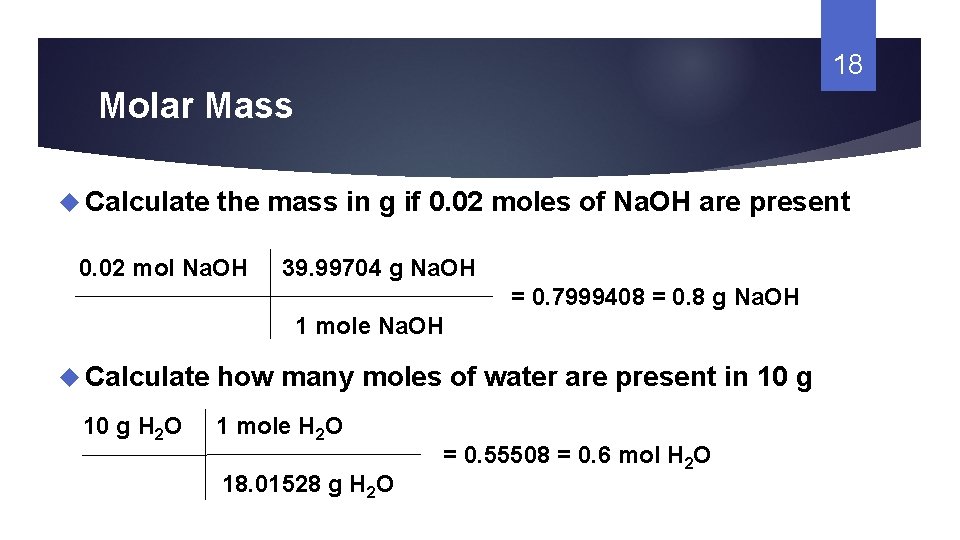 Source: slidetodoc.com
Source: slidetodoc.com
Atomic weight of N a O H is 4 0 gmmol. Type in your own numbers in the form to convert the units. So005 moles 40005 g 2g. The compound sodium hydroxide NaOH comprises the atoms. How many moles are in 1 gram.
 Source: youtube.com
Source: youtube.com
The weight of one mole is 84 grams. What mass of iron Fe is needed to react with 160 grams of sulfur S8. For 1000ml of 1N solution NaOH is needed 1moletherefore for 1000ml of 005N solution NaOH is needed 005 moles. So to prepare 1N solution of NaOH we need to dissolve 40 gms of NaOH in 1000 ml of distilled water. Molar mass of NaOH is 40 gmol.
 Source: slideplayer.com
Source: slideplayer.com
2g NaOH is needed for 1000ml solution. So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles. 160 g NaOH x 1 mol NaOH x 1 mol Na2O 200 mol Na2O 4000 g NaOH 2 mol NaOH 5. Add these up and you get 40 grams per mole of NaOH. One moles of sodium hydroxide 40 gm of sodium hydroxide.
 Source: oneclass.com
Source: oneclass.com
If want prepare 1 molar NaOH solution then we need 40 gm NaOH dissolve in one liter of water so it became one 1 molar NaOH solution. Take the number of moles and multiply it by the atomic mass. If playback doesnt begin shortly try restarting your device. 160 g NaOH x 1 mol NaOH x 1 mol Na2O 200 mol Na2O 4000 g NaOH 2 mol NaOH 5. Atomic weight of N a O H is 4 0 gmmol.
 Source: youtube.com
Source: youtube.com
2g NaOH is needed for 1000ml solution. The final calculation is 120 x 2 x 40 62 1548387. The technique used can be applied to any mole to gram conversion. How many grams of NaOH should be added t. What mass of iron Fe is needed to react with 160 grams of sulfur S8.
 Source: oneclass.com
Source: oneclass.com
Divide by one mole for units to cancel. Add these up and you get 40 grams per mole of NaOH. Now we can obtain the number of mole of Na needed to produce 40g of NaOH as follow. So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles. 160 g S8 x 1 mol S8 x 8 mol Fe x 5585 g Fe 27.

To prepare 2N solution of NaOH we must dissolve 24080 gms of NaOH in 1000 ml of distilled water. 1 mole is equal to 1 moles NaOH or 3999711 grams. The final calculation is 120 x 2 x 40 62 1548387. Divide by one mole for units to cancel. Therefore Xmol of Na will react to produce 40g of NaOH ie.
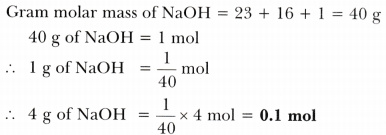 Source: ask.learncbse.in
Source: ask.learncbse.in
Sodium oxygen and hydrogen. You can view more details on each measurement unit. To prepare 2N solution of NaOH we must dissolve 24080 gms of NaOH in 1000 ml of distilled water. Their respective atomic weights are. View solution If 2 0 0 ml solution of 0.
 Source: slideplayer.com
Source: slideplayer.com
Since sodium hydroxide reacts 11 with the KHP acid this also the number of moles of KHP needed for a complete reaction and neutralization. Molar mass of NaOH is 40 gmol. View solution How many grams of N a O H must be added to make 2 0 0 m L of a 1 M N a O H solution. How many moles are in 1 gram. Type in your own numbers in the form to convert the units.
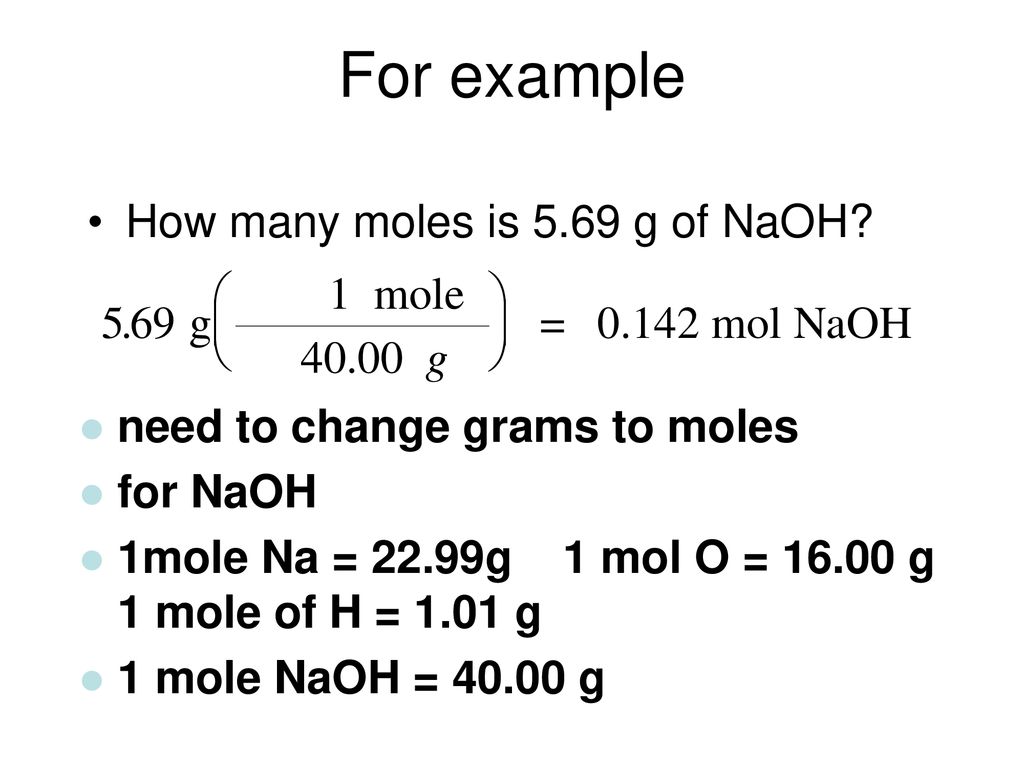 Source: slideplayer.com
Source: slideplayer.com
Type in your own numbers in the form to convert the units. Note that rounding errors may occur so always check the results. For 1000ml of 1N solution NaOH is needed 1moletherefore for 1000ml of 005N solution NaOH is needed 005 moles. NaOH400 grams540 moles NaOH Atilde. The answer is 3999711.
 Source: bartleby.com
Source: bartleby.com
Atomic weight of N a O H is 4 0 gmmol. 1 mole is equal to 1 moles NaOH or 3999711 grams. The answer is 3999711. If want prepare 1 molar NaOH solution then we need 40 gm NaOH dissolve in one liter of water so it became one 1 molar NaOH solution. Atomic weight of N a O H is 4 0 gmmol.
 Source: slideplayer.com
Source: slideplayer.com
If want prepare 1 molar NaOH solution then we need 40 gm NaOH dissolve in one liter of water so it became one 1 molar NaOH solution. Now we can obtain the number of mole of Na needed to produce 40g of NaOH as follow. The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer. Videos you watch may be added to the TVs watch history and influence TV recommendations. Na is 23 gmol O is 16 gmol and H is 1 gmol.
 Source: clutchprep.com
Source: clutchprep.com
Videos you watch may be added to the TVs watch history and influence TV recommendations. The compound sodium hydroxide NaOH comprises the atoms. How many moles are in 100g of NaOH. 1 molarity and dilutions issues. Therefore Xmol of Na will react to produce 40g of NaOH ie.
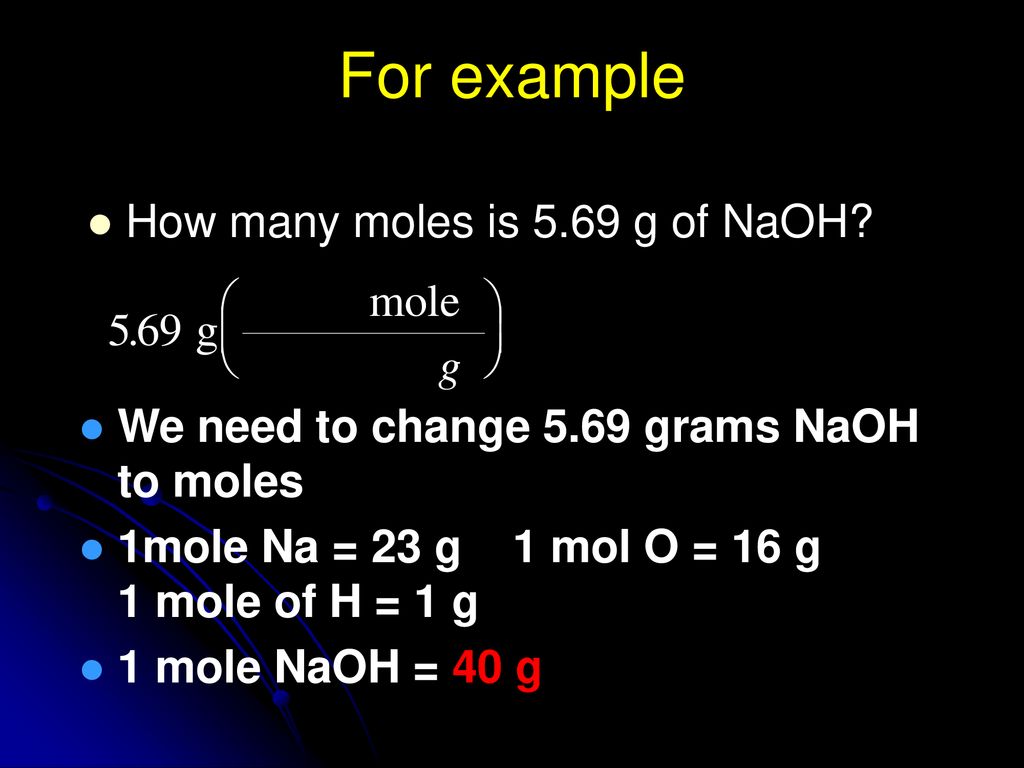 Source: slideplayer.com
Source: slideplayer.com
How many moles of Na2O are required to produce 160 grams of NaOH 4000 gmol. Sodium oxygen and hydrogen. 1 molarity and dilutions issues. Add these up and you get 40 grams per mole of NaOH. So we can said.
 Source: slideplayer.com
Source: slideplayer.com
NaOH400 grams540 moles NaOH Atilde. 8 Fe S8 8 FeS. The technique used can be applied to any mole to gram conversion. Look at the periodic table. If playback doesnt begin shortly try restarting your device.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is 1 mole of naoh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






