Your How many grams is 1 mole of nacl images are ready. How many grams is 1 mole of nacl are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams is 1 mole of nacl files here. Find and Download all royalty-free vectors.
If you’re searching for how many grams is 1 mole of nacl images information linked to the how many grams is 1 mole of nacl interest, you have pay a visit to the right blog. Our website always gives you hints for downloading the maximum quality video and image content, please kindly surf and find more enlightening video articles and graphics that match your interests.
How Many Grams Is 1 Mole Of Nacl. 1 grams NaCl is equal to 0017110756386119 mole. 124 1024 formula units. Molar mass is the amount grams that one mole weighs. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams.

One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. Hence 1 litre would contain. So the MrNaCl 23 355 585. Molar mass of NaCl. Molar mass of NaCl 5844277 gmol. One mole of sodium will make one mole of sodium chloride.
1 mole is equal to 1 moles NaCl or 5844277 grams.
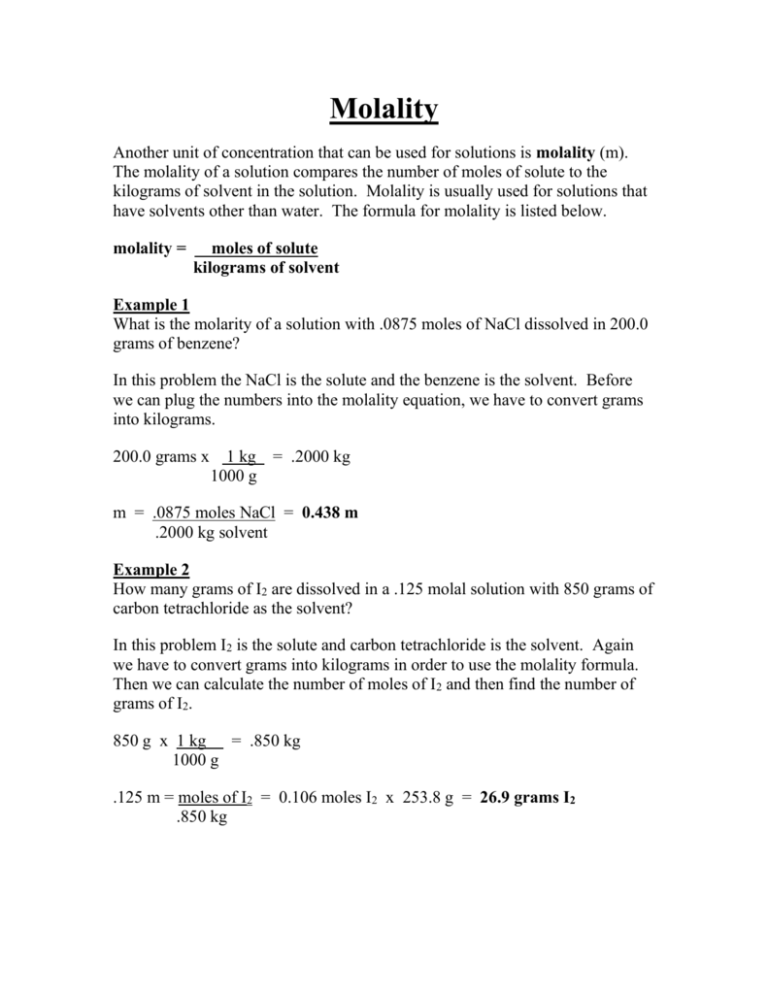
Click hereto get an answer to your question How many moles and how many grams of NaCl are present in 250 mL of a 05 M NaCl solution. Molecular weight of NaCl or mol This compound is also known as Sodium Chloride. Track your food intake exercise sleep and meditation for free. The molecular weight of sodium chloride NaCl is 5844 so one gram molecular weight 1 mole is 5844g. Note that rounding errors may occur so always check the results. The answer is 5844277.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass is the amount grams that one mole weighs. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. This compound is also known as Sodium Chloride. 5844 x 002 11688 g However there are only 350 ml which is 035 liters. You need to find the molar mass of NaCl which is the same as the amu on the periodic table in grams.

Sodium chloride has a molecular mass of 5844 so a mole of NaCl is 5844 grams. Convert between NaCl weight and moles. We do this by looking up the relative atomic masses of Na and Cl in the periodic table. Molecular weight of NaCl or mol This compound is also known as Sodium Chloride. So it is 2299 Na 3545 Cl 5844.
 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
Molar mass of NaCl is 584428 gmol. Note that rounding errors may occur so always check the results. 1 mol of NaCl 602 1023 formula units. From the Periodic Table you can see that Na is 2299 gmole and Cl is 3545 gmole. Hence 1 litre would contain.
 Source: youtube.com
Source: youtube.com
Use this page to learn how to convert between moles NaCl and gram. Molar mass of NaCl is 584428 gmol. One molecule of water H2O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a mole of water molecules would weigh 1802 grams. The answer is 5844277. Formula units NaCl 120 g NaCl 1 mol NaCl 5844 g NaCl 602 1023 formula units 1 mol NaCl.
 Source: youtube.com
Source: youtube.com
NaCl So four moles of sodium will make four. Then multiply by the quantity 14 liter 250 cm3 to get 18 moles NaCl. What is the mole equation. Molecular weight of NaCl or mol This compound is also known as Sodium Chloride. Next to get the moles of glucose we must multiply 54 g by the inverse of 18012 gmol which is 1 mol18012 g to get our answer in moles.
 Source: youtube.com
Source: youtube.com
One mole of sodium will make one mole of sodium chloride. The molecular weight of chlorine Cl is 3545 grams. The molecular weight of sodium Na is 2299 grams. A 002 M solution contains 002 moles per liter. What number of moles of NaCl are wanted to arrange 150 liters of 0250 M answer.
 Source: slideplayer.com
Source: slideplayer.com
NaCl So four moles of sodium will make four. 1 mol of NaCl 602 1023 formula units. A 002 M solution contains 002 moles per liter. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. Convert between NaCl weight and moles.
 Source: pinterest.com
Source: pinterest.com
For our practice problem wel. This compound is also known as Sodium Chloride. From the Periodic Table you can see that Na is 2299 gmole and Cl is 3545 gmole. 1 mole is equal to 1 moles NaCl or 5844277 grams. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams.
 Source: pinterest.com
Source: pinterest.com
Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. Molar mass is the amount grams that one mole weighs. NaCl is an ionic compound so there are no molecules only formula units. One mole of sodium will make one mole of sodium chloride. Then multiply by the quantity 14 liter 250 cm3 to get 18 moles NaCl.
 Source: slideplayer.com
Source: slideplayer.com
You multiply the grams they provide you 25 g by utilising the ratio of a million mole over the molar mass of NaCl. The SI base unit for amount of substance is the mole. You multiply the grams they provide you 25 g by utilising the ratio of a million mole over the molar mass of NaCl. The technique used can be applied to any mole to gram conversion. Next to get the moles of glucose we must multiply 54 g by the inverse of 18012 gmol which is 1 mol18012 g to get our answer in moles.
 Source: studylib.net
Source: studylib.net
What number of moles of NaCl are wanted to arrange 150 liters of 0250 M answer. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. Na Cl. 1 mol of NaCl 602 1023 formula units. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. 1 grams NaCl is equal to 0017110756386119 mole. The concentration of 05 molar NaCl is half a mole per liter. Jul 18 2012 17. NaCl is approximately 58 grams per mole so the amount of salt NaCl in the sample would be around 725 grams.

Get control of 2021. Click hereto get an answer to your question How many moles and how many grams of NaCl are present in 250 mL of a 05 M NaCl solution. Molar mass is the amount grams that one mole weighs. As stated 1 mole of NaCl weighs 5844 g. 1 grams NaCl is equal to 0017110756386119 mole.
 Source: pinterest.com
Source: pinterest.com
Na has an atomic mass of 23 and Cl is 355. Use this page to learn how to convert between moles NaCl and gram. If you dissolve 5844g of NaCl in a final volume of 1 litre you have made a 1M NaCl solution. Compound name is sodium chloride. You can view more details on each measurement unit.

How many moles are there in 50g NaCl. 1 mole 60221023 6022 10 23 atoms molecules protons etc. 1946 moles would be 1946x584428 or 11373 grams. Molar mass of NaCl is 584428 gmol. NaCl is an ionic compound so there are no molecules only formula units.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is 1 mole of nacl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.