Your How many grams is 1 mole of h20 images are ready in this website. How many grams is 1 mole of h20 are a topic that is being searched for and liked by netizens today. You can Get the How many grams is 1 mole of h20 files here. Find and Download all royalty-free photos.
If you’re searching for how many grams is 1 mole of h20 pictures information connected with to the how many grams is 1 mole of h20 keyword, you have come to the ideal site. Our website always gives you suggestions for seeing the highest quality video and picture content, please kindly search and locate more enlightening video articles and graphics that match your interests.
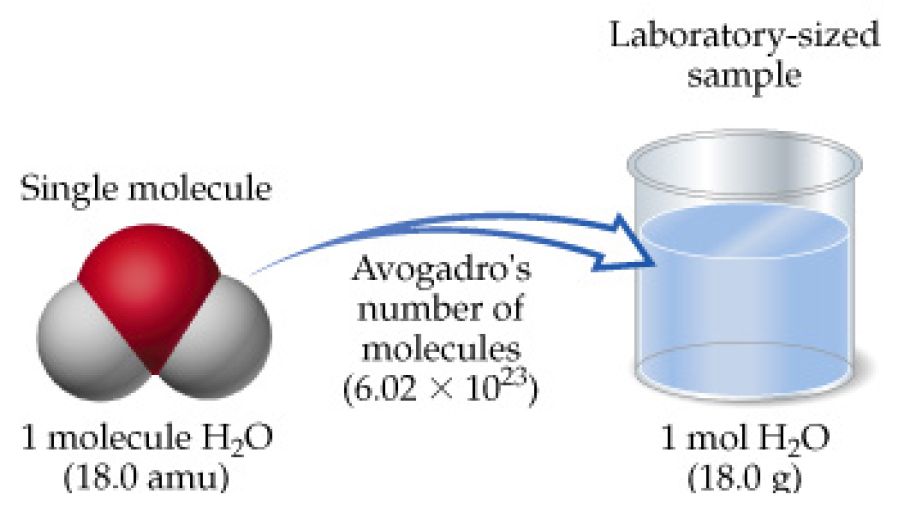
How Many Grams Is 1 Mole Of H20. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 10 23 oxygen atoms. You want to calculate how many moles of H2 you have. We assume you are converting between moles In and gram. 45 grams H20 x 1 mole H2018 grams H2O x 602E23 molecules H201 mole H2O the grams H2O and moles H2O cancel out.
 Ppt The Mole Powerpoint Presentation Free Download Id 6795730 From slideserve.com
Ppt The Mole Powerpoint Presentation Free Download Id 6795730 From slideserve.com
Correct to the nearest integer. 1 mole of oxygen atoms got 16 grams. In this video well learn to convert moles of H2O to grams. So is equal to 18 moles. Mass 30g so. 45 grams H20 x 1 mole H2018 grams H2O x 602E23 molecules H201 mole H2O the grams H2O and moles H2O cancel out.
The technique used can be applied to any mole to gram conversion.
9 is 12 of 18 meaning that 12 mole is present in 9 g of water. To calculate the GFM you require a data booklet or similar. One mole of water has a mass of 18 grams and a volume of 18 mL as the density of water is 1 g per cm3. Thus 36g of water is equivalent to 12 1024molecules. Because the molecular mass of Hydrogen is 1grammole there is 1 mole of hydrogen in 1 gram of hydrogen atoms. Mole of oxygen gas to produce.
 Source: clutchprep.com
Source: clutchprep.com
200mol 602 1023molecules 1mol 1204 1024molecules. How many hydrogen atoms are in 100g water. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 10 23 oxygen atoms. You can view more details on each measurement unit. Use this page to learn how to convert between grams H20 and mole.
 Source: slideplayer.com
Source: slideplayer.com
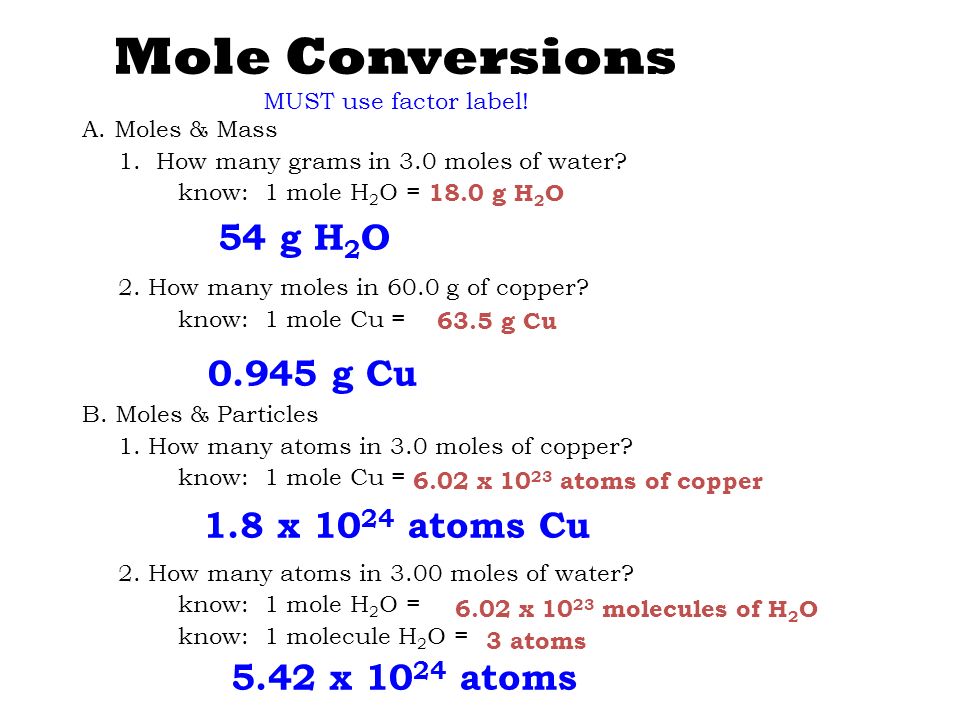
45 grams H20 x 1 mole H2018 grams H2O x 602E23 molecules H201 mole H2O the grams H2O and moles H2O cancel out. Thus 1 litre of water contains 1 18 x 1000 x 6 x 123 molecules assume you can do the maths. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. Moles H 2 100 g H 2 x 1 mole 495 mol H 2 available 202 g Moles O 2 750 g O 2x 1 mole 234 mol O 2 available 320 g Method 1 Limiting Reactants Chapter 4 moles O 2 needed 495 mol H 2 x 1 mol O 2 2 mol H 2 248 moles O 2 Step 2 Pick H 2 and find the moles O 2 needed to react with all of the H 2 Limiting Reactants Chapter 4 Step 3. One gram of H20 is close to 006 moles.
 Source: slideplayer.com
Source: slideplayer.com
Moles H 2 100 g H 2 x 1 mole 495 mol H 2 available 202 g Moles O 2 750 g O 2x 1 mole 234 mol O 2 available 320 g Method 1 Limiting Reactants Chapter 4 moles O 2 needed 495 mol H 2 x 1 mol O 2 2 mol H 2 248 moles O 2 Step 2 Pick H 2 and find the moles O 2 needed to react with all of the H 2 Limiting Reactants Chapter 4 Step 3. 9 is 12 of 18 meaning that 12 mole is present in 9 g of water. This tells you that the reaction produces twice as many moles of water as you have moles of oxygen gas that take part in the reaction. You want to calculate how many moles of H2 you have. In this video well learn to convert moles of H2O to grams.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of In or grams The SI base unit for amount of substance is the mole. How many is it. Chemistry questions and answers. Using the moles of H 2O that were just obtained we can use Avogrados number to perform dimensional analysis to cancel out units of mol to end up with molecules. 9 is 12 of 18 meaning that 12 mole is present in 9 g of water.

What is the mass of one mole of Br2 atoms. Assuming that hydrogen gas is not a limiting reagent you can say that the reaction will produce. 1 mole is equal to 1 moles H20 or 201588 grams. 1 grams H20 is equal to 004960612734885 mole. Chemistry questions and answers.
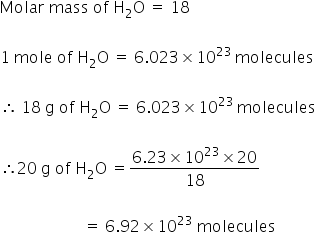 Source: topperlearning.com
Source: topperlearning.com
You know that your reaction uses. Note that rounding errors may occur so always check the results. One mole of water has a mass of 18 grams and a volume of 18 mL as the density of water is 1 g per cm3. How many moles are in a gram. What is the mass of one mole of Br2 atoms.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles NaCl or 5844277 grams. Correct to the nearest integer. Thus 1 litre of water contains 1 18 x 1000 x 6 x 123 molecules assume you can do the maths. Answer 1 of 4. 20 g of hydrogen gas is 10 moles of H2 which is 20 moles of H atoms.
 Source: chegg.com
Source: chegg.com
So is equal to 18 moles. What is the mass of 37 moles of Br2. So is equal to 18 moles. We assume you are converting between moles NaCl and gram. 6021023 The Attempt at a Solution I got to the equation.
 Source: youtube.com
Source: youtube.com
Correct to the nearest integer. 1 mole of oxygen atoms got 16 grams. To calculate the GFM you require a data booklet or similar. What is the number of moles of h20 produced if you combust 5 of CH4 according to the following balanced equation. You know that your reaction uses.
 Source: slideplayer.com
Source: slideplayer.com
Using the moles of H 2O that were just obtained we can use Avogrados number to perform dimensional analysis to cancel out units of mol to end up with molecules. How many h20 molecules are in 9 grams of water. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 10 23 oxygen atoms. 12 6022 1023 3011 1023 molecules H2O. 1 grams H20 is equal to 004960612734885 mole.
 Source: slideplayer.com
Source: slideplayer.com
Assuming that hydrogen gas is not a limiting reagent you can say that the reaction will produce. Use this page to learn how to convert between grams H20 and mole. The answer is 0017110756386119. How many moles are in a gram. Mole of oxygen gas to produce.
 Source: slideserve.com
Source: slideserve.com
1 Limiting reactant LR is one with higher molar mass 2 A is LR. What is the mass of one mole of Br2 atoms. Correct to the nearest integer. 1 grams H20 is equal to 004960612734885 mole. 1 Limiting reactant LR is one with higher molar mass 2 A is LR.
 Source: socratic.org
Source: socratic.org
How many h20 molecules are in 9 grams of water. We assume you are converting between moles In and gram. Assuming that hydrogen gas is not a limiting reagent you can say that the reaction will produce. Chemistry questions and answers. 1 mole is equal to 1 moles NaCl or 5844277 grams.
 Source: youtube.com
Source: youtube.com
How many atoms are in 08288 grams of sodium. How many moles are in a gram. 20 g of hydrogen gas is 10 moles of H2 which is 20 moles of H atoms. The answer is 1665253. 37 mol Br2 x 1598 gmol 59126 g Br2 27.
 Source: slideplayer.com
Source: slideplayer.com
How many grams of C2H42806 gmol are needed to produce 370 moles of water. 20 g of hydrogen gas is 10 moles of H2 which is 20 moles of H atoms. To calculate the GFM you require a data booklet or similar. ___ mol C2H4 287 g Fe. 1 mole is equal to 1 moles NaCl or 5844277 grams.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is 1 mole of h20 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.