Your How many grams is 1 mole of aluminum chloride images are available. How many grams is 1 mole of aluminum chloride are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams is 1 mole of aluminum chloride files here. Download all royalty-free vectors.
If you’re looking for how many grams is 1 mole of aluminum chloride images information connected with to the how many grams is 1 mole of aluminum chloride keyword, you have come to the right blog. Our website always gives you hints for downloading the highest quality video and picture content, please kindly hunt and find more informative video articles and images that fit your interests.
How Many Grams Is 1 Mole Of Aluminum Chloride. 1 mole is equal to 1 moles aluminum chloride or 133340538 grams. 1 mole is equal to 1 moles Aluminium Chloride or 133340538 grams. As 2 moles of aluminium chloride obtained from 3 moles of chlorine. This compound is also known as Magnesium Chloride.
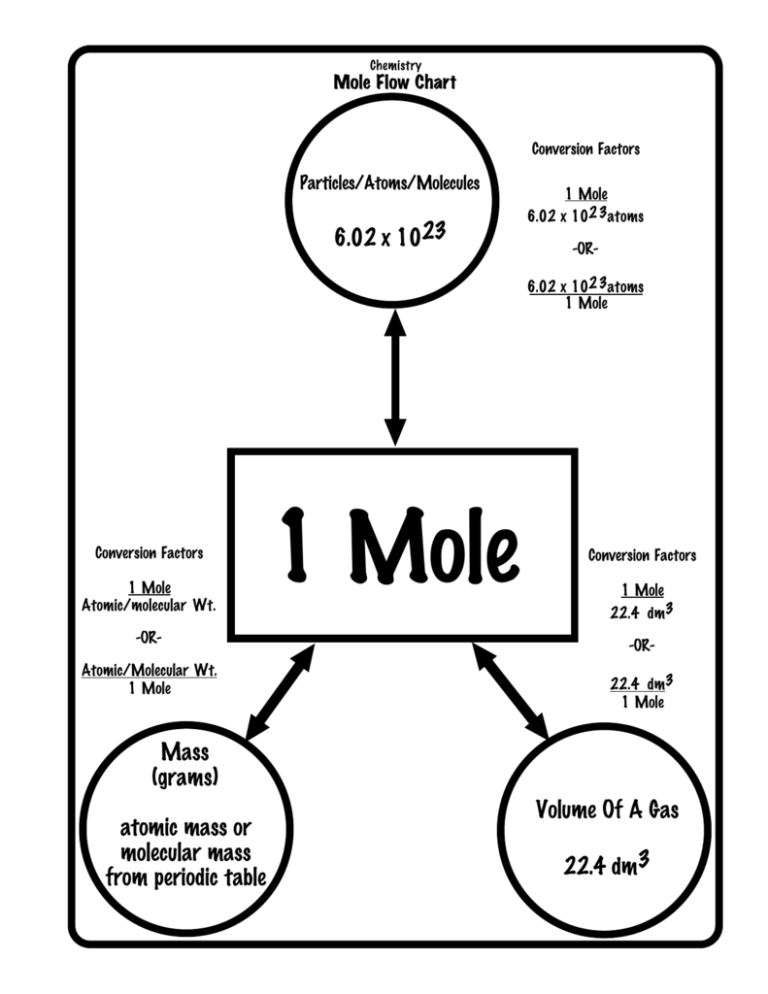
 The Mole The Mole The Mole Memorize This Number 1 Mol 6 02 X 1023 Of Something A Mole Is Defined As The Number Of Particles In Exactly 12g Of Carbon Ppt Download From slideplayer.com
The Mole The Mole The Mole Memorize This Number 1 Mol 6 02 X 1023 Of Something A Mole Is Defined As The Number Of Particles In Exactly 12g Of Carbon Ppt Download From slideplayer.com
1 mole is equal to 1 moles aluminum chloride or 133340538 grams. Note that rounding errors may occur so always check the results. Mol-1 033 moles. 13333 gmole How many moles are in AlCl3. 1 mole is equal to 1 moles Aluminium Chloride or 133340538 grams. Thus the total number of moles in 9 grams of aluminium 9g27g.
1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams.
The answer is 5349146. 13333 gmole How many moles are in AlCl3. The chemical formula for aluminum chloride is AlCl₃. How many grams of aluminum will react with 34 moles of chlorine. Given Moles of aluminum chloride 2 moles. The SI base unit for quantity of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
Therefore 1 mole of aluminium will weigh 27 grams. 21 molesliter 0655 L 0013755 moles AlCl3 013755 3 moles Cl 1 mole AlCl3 0041265 moles Cl avagadros number number of chloride ions How many moles of O2- ions are in 065 moles. The number of moles in 0255 g is. Aluminium Chloride molecular weight. Therefore moles of chloride ions present in it are as follows.
 Source: slidetodoc.com
Source: slidetodoc.com
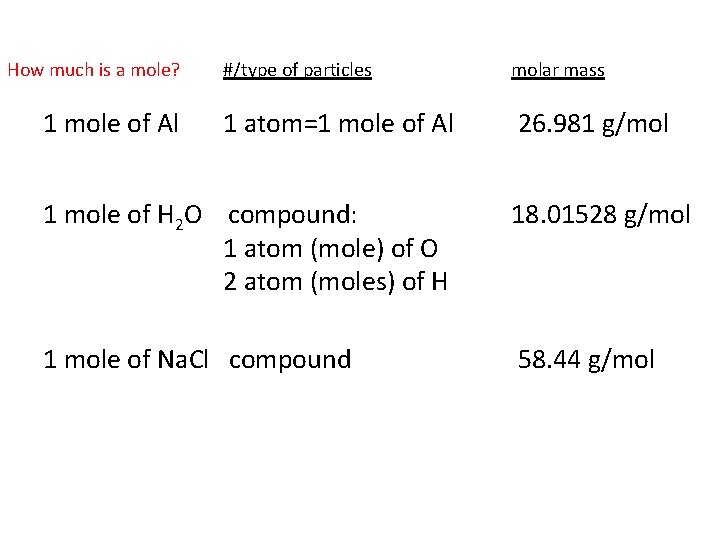
The SI base unit for amount of substance is the mole. 270 g Al 1 mol Al 612 g Al e. The balanced chemical reaction of the formation of aluminum chloride will be From the balanced chemical reaction we conclude that. The SI base unit for amount of substance is the mole. What number of grams are in a single mole of aluminum chloride.
 Source: slideplayer.com
Source: slideplayer.com
What number of moles of chloride ions are in 02560g of aluminum chloride. What is the formula for Aluminium chloride. Basically its the amount of atomic mass units required to equal 1 gram of mass. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram. The chemical formula for aluminum chloride is AlCl₃.
 Source: slidetodoc.com
Source: slidetodoc.com
21 molesliter 0655 L 0013755 moles AlCl3 013755 3 moles Cl 1 mole AlCl3 0041265 moles Cl avagadros number number of chloride ions How many moles of O2- ions are in 065 moles. Each molecule has 2 atoms so multiply this number by 2. Aluminum hydroxide is an ingredient in some antacids. 1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams. The SI base unit for amount of substance is the mole.
 Source: slidetodoc.com
Source: slidetodoc.com
1 grams AlCl3 is equal to 00074995947593972 mole. How many moles of chloride ions are in 00750 g of Aluminium chloride. Mol-1 033 moles. Note that rounding errors may occur so always check the results. You can view more details on each measurement unit.
 Source: slidetodoc.com
Source: slidetodoc.com
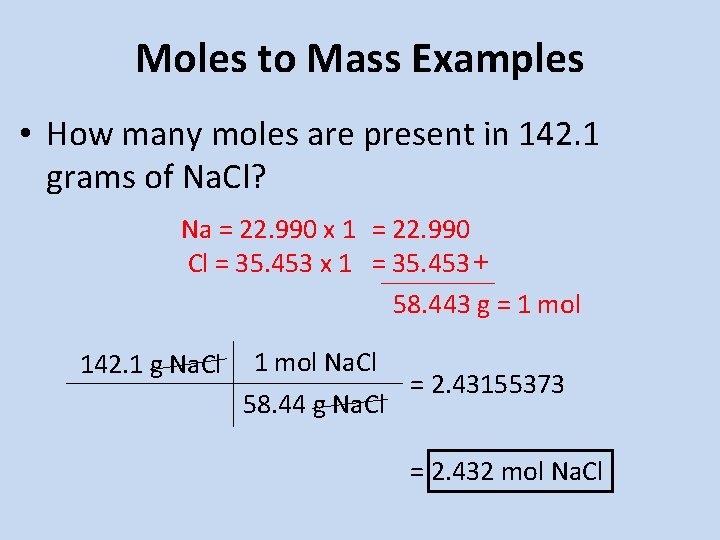
The number of moles in 0255 g is. 1023 1 mole Al 22. 0255 g13334 gmol 000191 mole. To convert between grams and moles you would use the substances molar mass. The chemical formula for aluminum chloride is AlCl₃.
 Source: slideplayer.com
Source: slideplayer.com
Therefore moles of chloride ions present in it are as follows. Given Moles of aluminum chloride 2 moles. Al OH3 s HCl aq AlCl3 aq H2O 1 A. Balance the equation first. 1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams.
 Source: slideplayer.com
Source: slideplayer.com
The chemical formula for aluminum chloride is AlCl₃. How many grams of aluminum will react with 34 moles of chlorine. How many grams Ammonium Chloride in 1 mol. To convert between grams and moles you would use the substances molar mass. 1 mole is equal to 1 moles aluminum chloride or 133340538 grams.
 Source: studylib.net
Source: studylib.net
The molecular formula for aluminum chloride is AlCl3. 000191 moles AlCl3 X 3 chloridesmole of AlCl3 000573 moles of chloride. As 2 moles of aluminium chloride obtained from 3 moles of chlorine. How many moles are in aluminum chloride. 10 grams aluminum 1 mole Al2698 grams6022.
 Source: pinterest.com
Source: pinterest.com
Mol AlCl 3 17 g Al. How many grams are in 300 moles of aluminum. Now if we are talking 0255 g then we have to determine how many moles this corresponds to. Unit 6 Moles and Options Homework Moles Follow 1. 13333 gmole How many moles are in AlCl3.
 Source: slideplayer.com
Source: slideplayer.com
2 mol Al 3 mol Cl 2. The answer is 133340538. The SI base unit for amount of substance is the mole. 000191 moles AlCl3 X 3 chloridesmole of AlCl3 000573 moles of chloride. The molecular formula for Aluminium Chloride is AlCl3.
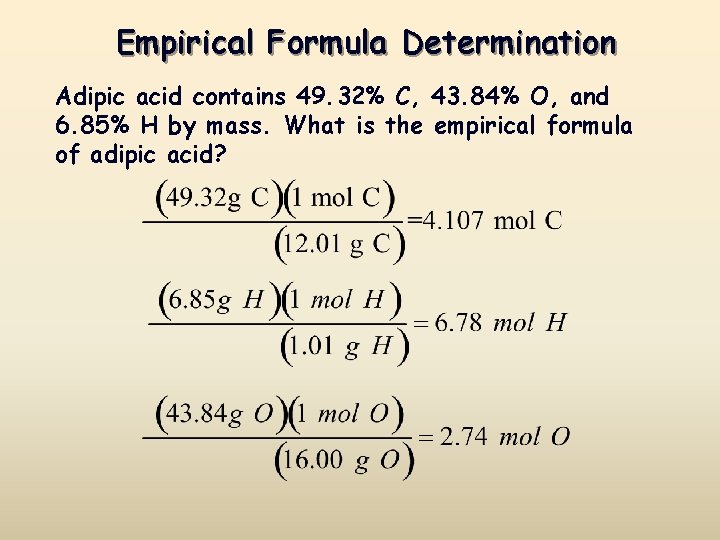 Source: slidetodoc.com
Source: slidetodoc.com
Thus the total number of moles in 9 grams of aluminium 9g27g. 13333 gmole How many moles are in AlCl3. Use this page to learn how to convert between moles aluminum chloride and gram. 0255 g13334 gmol 000191 mole. 1 mol AlCl 3 1335 g AlCl 3.
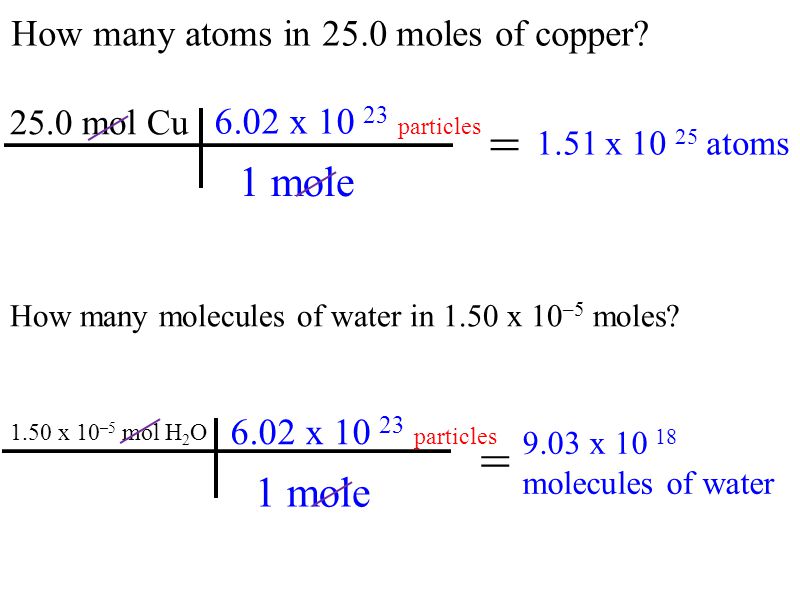 Source: slideplayer.com
Source: slideplayer.com
000191 moles AlCl3 X 3 chloridesmole of AlCl3 000573 moles of chloride. We assume you are converting between grams Ammonium Chloride and mole. Hence we can conclude that there are 00016 mol of chloride ions present in 00750 g of. Balance the equation first. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
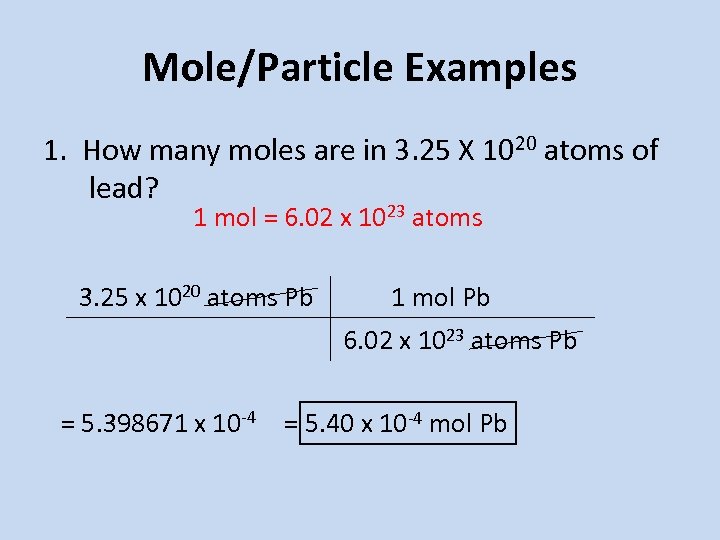
1023 1 mole Al 22. Therefore 1 mole of aluminium will weigh 27 grams. How many moles are in aluminum chloride. 1 mole is equal to 1 moles aluminum chloride or 133340538 grams. It reacts with hydrochloric acid according to the following equation.
 Source: present5.com
Source: present5.com
Therefore 1 mole of aluminium will weigh 27 grams. This compound is also known as Magnesium Chloride. 0255 g13334 gmol 000191 mole. The mass of chlorine required are 213 grams. We assume you are converting between grams aluminum chloride and mole.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is 1 mole of aluminum chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






