Your How many grams is 1 mole hcl images are available. How many grams is 1 mole hcl are a topic that is being searched for and liked by netizens now. You can Download the How many grams is 1 mole hcl files here. Download all free images.
If you’re searching for how many grams is 1 mole hcl pictures information related to the how many grams is 1 mole hcl keyword, you have pay a visit to the right site. Our site frequently provides you with hints for viewing the maximum quality video and picture content, please kindly surf and locate more enlightening video content and graphics that match your interests.
How Many Grams Is 1 Mole Hcl. Mass in grammolesmolecular mass. Punineep and 4 more users found this answer helpful. One mole of HCl365grams. Molecular weight of HCl or grams This compound is also known as Hydrochloric Acid.
 Molarity A Measurement Of The Concentration Of A Solution Molarity M Is Equal To The Moles Of Solute N Per Liter Of Solution M N V Mol L Calculate Ppt Download From slideplayer.com
Molarity A Measurement Of The Concentration Of A Solution Molarity M Is Equal To The Moles Of Solute N Per Liter Of Solution M N V Mol L Calculate Ppt Download From slideplayer.com
This compound is also known as Hydrochloric Acid. You can view more details on each measurement unit. The answer is 0017110756386119. 1 mole is equal to 1 moles HCl or 3646094 grams. Al2S3 6 HCl 2 AlCl3 3 H2S. Density of hydrochloric acid is equal to 16423 kgm³.
What is 1N HCl.
Therefore we can say that 1 liter of Hydrochloric acid contains 12178 moles of HCl or in other words molarity of 37 ww Hydrochloric acid is equal to 12178 M. Hydrochloric acid weighs 00016423 gram per cubic centimeter or 16423 kilogram per cubic meter ie. Use this page to learn how to convert between moles HCl and gram. So all we need to know is moles of NaOH 4g 40 gmole 01 moles. Density of hydrochloric acid is equal to 16423 kgm³. The correct answer is 1.
 Source: slideplayer.com
Source: slideplayer.com
1191g solution 37 g HCl 100g solution 44067 g HCl. This means that your sample will contain. 1 mole is equal to 1 moles HCl or 3646094 grams. 027 mole of hydrogen needed to produce 20 gram of hydrochloric acid HCl. No3n has oxidation number xo has oxidation number -2.
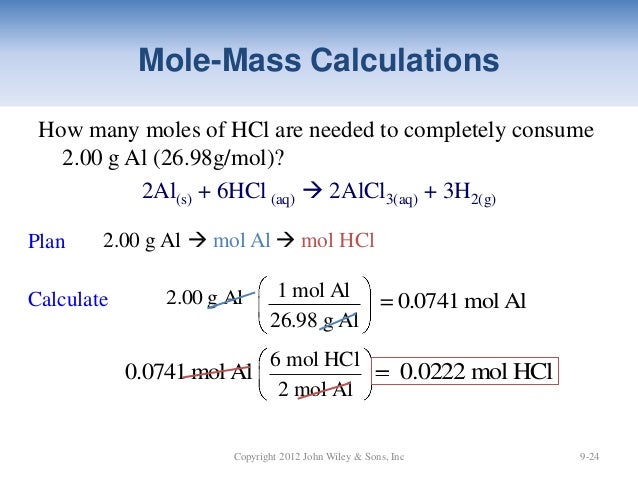 Source: slideshare.net
Source: slideshare.net
N 5988 g 18015 gmol 3324 mol. Molar mass of HCl 3646094 gmol. What is the mass of 32 moles of milk of magnesia. Molar mass HCl 3646gmol The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. Now you know that the solution has a 37 concentration by mass which means that every 100 g of solution will contain 37 g of hydrochloric acid.
 Source: slideplayer.com
Source: slideplayer.com
So all we need to know is moles of NaOH 4g 40 gmole 01 moles. N2 3 H 2 2 NH 3 Note. From where we have 1 mole of Al2S3 reacting with 6. Molecular mass of HCl 365gms. We can also solve it by balance equation.
 Source: youtube.com
Source: youtube.com
Mol NH 3 Conversion. We assume you are converting between moles NaCl and gram. 10 mol H 2 Find. What is its molecular formula. 44401776 grams of Hydrogen chloride will be equal to 444.
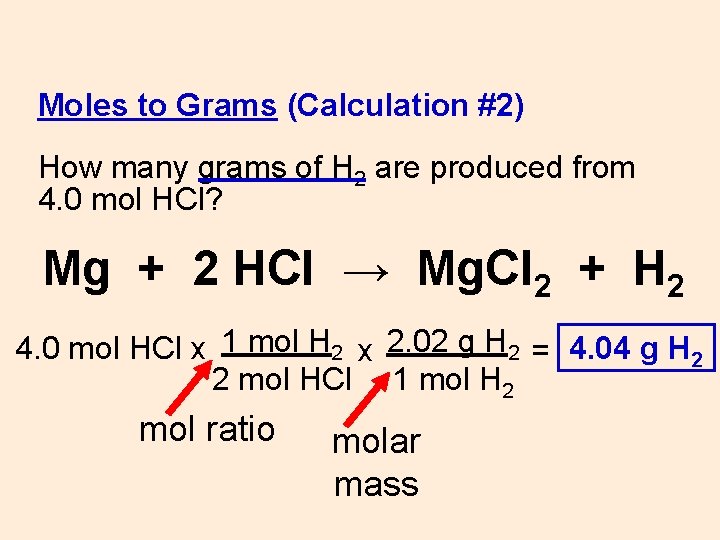 Source: slidetodoc.com
Source: slidetodoc.com
Molar mass HCl 3646gmol The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. 1 g of HCl will be equal to 1 36. The correct answer would be b volume versus temperature because they are also directly proportional. X1 -23 charge of the nitrate ion and we know that charge of the nitrate ion -1so x1 -23 -1x-6-1x5answer a. How many moles of solute are in 250ml of 20 m CaCl2.
 Source: youtube.com
Source: youtube.com
How many moles of solute are present. Therefore we can say that 1 liter of Hydrochloric acid contains 12178 moles of HCl or in other words molarity of 37 ww Hydrochloric acid is equal to 12178 M. 44401776 grams of Hydrogen chloride will be equal to 444. Molar mass of HCl 3646094 gmol. Al2S3 6 HCl 2 AlCl3 3 H2S.
 Source: nagwa.com
Source: nagwa.com
As you already know how the grams to moles conversion work find the number of moles. To prepare a 1 M solution the ratio would be 1 moles of HCl per liter of water. 219 grams this is arrived at by considering the actual yield of the reacting compounds. The molar mass of oxygen. The correct answer would be b volume versus temperature because they are also directly proportional.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles HCl or 3646094 grams. Now you know that the solution has a 37 concentration by mass which means that every 100 g of solution will contain 37 g of hydrochloric acid. 46 12178 moles. Acompound has a molar mass of 9202 gramsmole and its percent composition is 304 nitrogen n and 696 oxygen o. 1 g of HCl will be equal to 1 36.
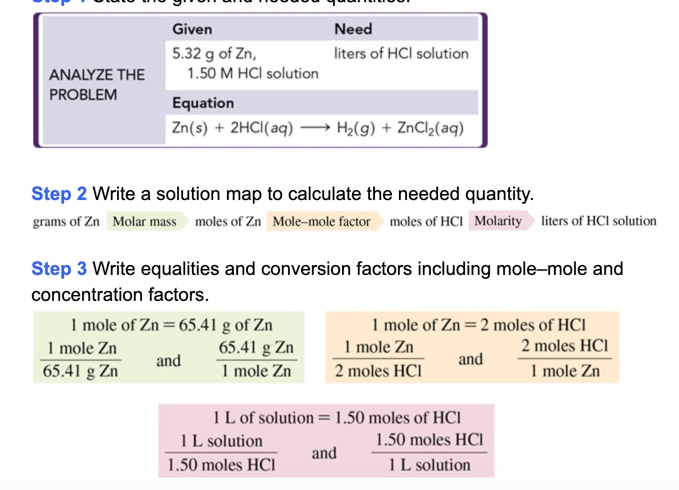 Source: chegg.com
Source: chegg.com
We assume you are converting between moles NaCl and gram. Molar mass HCl 3646gmol The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. 1191g solution 37 g HCl 100g solution 44067 g HCl. We can also solve it by balance equation. 219 grams this is arrived at by considering the actual yield of the reacting compounds.
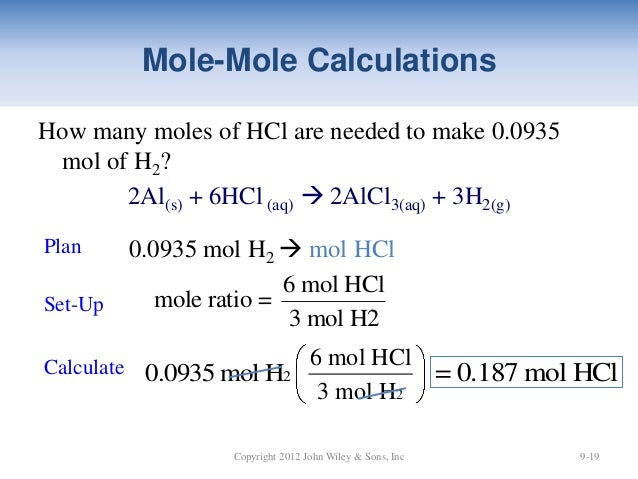 Source: slideshare.net
Source: slideshare.net
027 mole of hydrogen needed to produce 20 gram of hydrochloric acid HCl. This compound is also known as Sodium Chloride. Molar mass HCl 3646gmol The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. One mole of HCl365grams. 44401776 grams of Hydrogen chloride will be equal to 444.
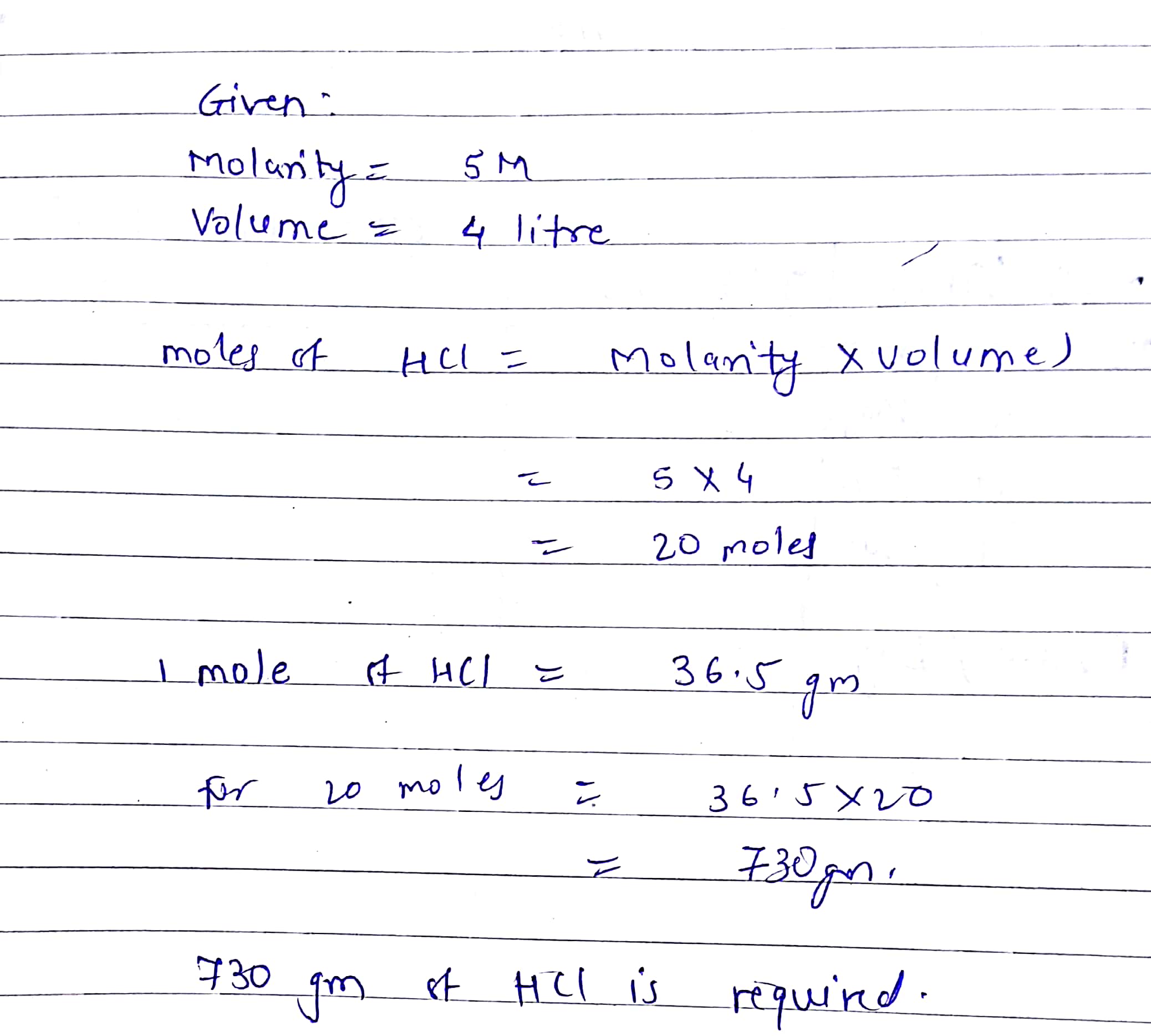 Source: topperlearning.com
Source: topperlearning.com
1 mole is equal to 1 moles NaCl or 5844277 grams. One mole of HCl365grams. The SI base unit for amount of substance is the mole. Now by cross multiplication. This is equal to 365 grams HCl.
 Source: toppr.com
Source: toppr.com
Molecular mass of HCl 365gms. Now by cross multiplication. Mol NH 3 Conversion. The answer is 0017110756386119. This is equal to 365 grams HCl.
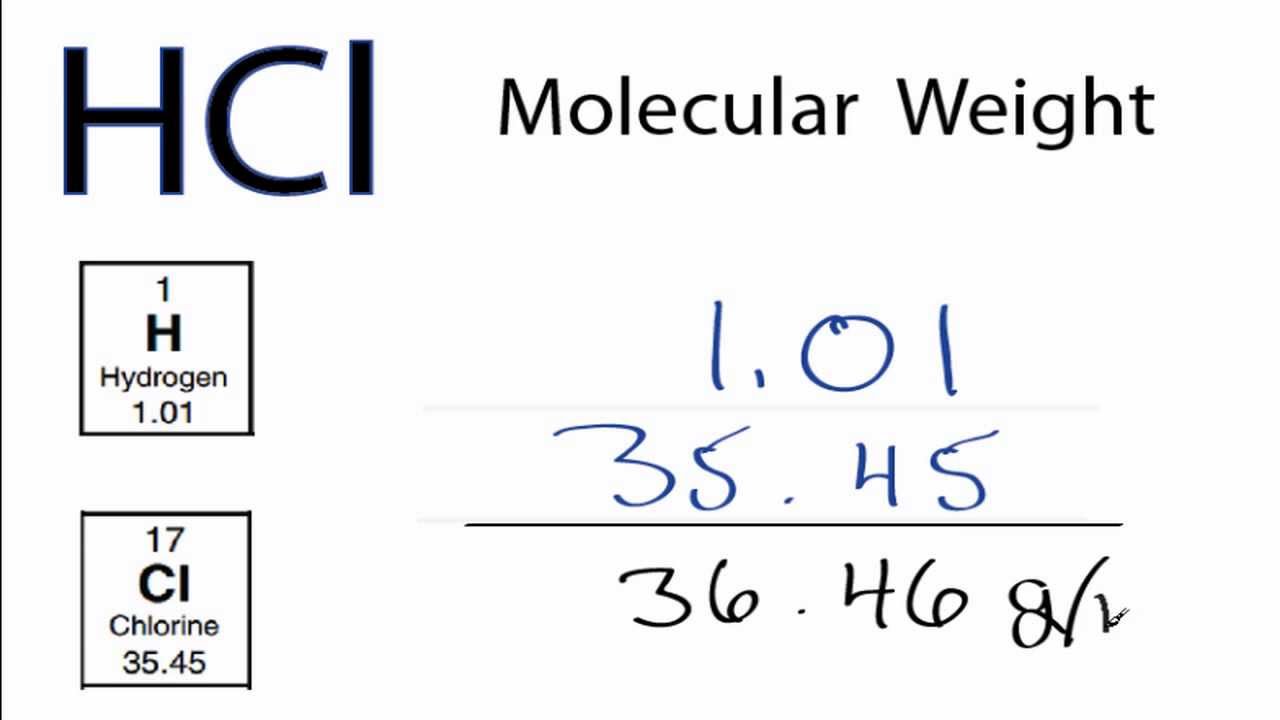 Source: youtube.com
Source: youtube.com
0132moles of HCl x. The answer is 0017110756386119. This means that your sample will contain. As you already know how the grams to moles conversion work find the number of moles. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
30 mol 3646 gmol 109 g HCl. The SI base unit for amount of substance is the mole. Density of hydrochloric acid is equal to 16423 kgm³. 46 12178 moles. How many grams are in 1 mole of HCl.
 Source: slideplayer.com
Source: slideplayer.com
This means that your sample will contain. This compound is also known as Sodium Chloride. To make 200 milliliters of your solution multiply gramsliter by liters needed. The molar mass of hydrogen is 10 grams per mole. We know that one mole of any substance is equal to its molecular mass.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is 1 mole hcl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






