Your How many grams is 1 mol of water images are available. How many grams is 1 mol of water are a topic that is being searched for and liked by netizens today. You can Download the How many grams is 1 mol of water files here. Find and Download all free photos and vectors.
If you’re searching for how many grams is 1 mol of water pictures information connected with to the how many grams is 1 mol of water keyword, you have pay a visit to the right site. Our site frequently provides you with hints for seeing the highest quality video and picture content, please kindly surf and find more enlightening video content and images that fit your interests.
How Many Grams Is 1 Mol Of Water. Fortunately this is another simple calculation. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. Water is composed of Hydrogen and Oxygen H2O. Molecular weight of Water or mol The molecular formula for Water is H2O.
 Gcse Chemistry Moles Calculations Teaching Resources Study Flashcards Chemistry Lessons Gcse Chemistry From pinterest.com
Gcse Chemistry Moles Calculations Teaching Resources Study Flashcards Chemistry Lessons Gcse Chemistry From pinterest.com
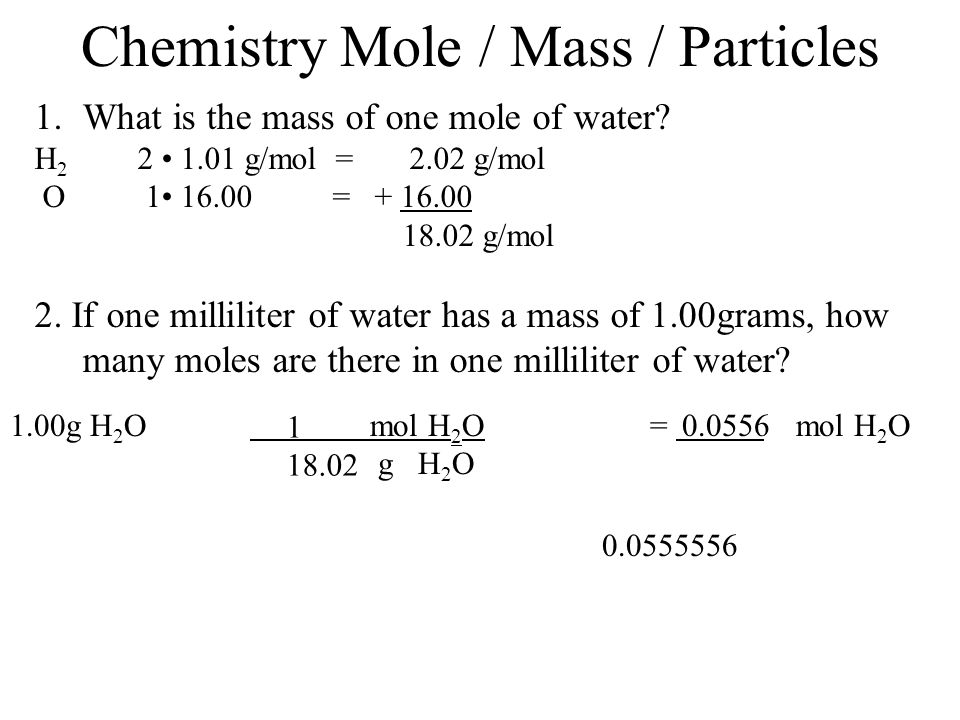
Note that rounding errors may occur so always check the results. A more precise value of the molar mass is 1801524 g if you want to re-calculate it. Molar mass C2H4 2805 gmol 0130mol 01302805 365g Your answer correct. 1 mole is equal to 1 moles Water or 1801528 grams. Note that rounding errors may occur so always check the results. This can also be written as 602210 23 mol-1The mass of one mole of a substance is equal to that substances molecular weight.
The mass of one mole of a substance is equal to that substances molecular weight.
Its easier to work with grams so convert the mass. Note that rounding errors may occur so always check the results. A more precise value of the molar mass is 1801524 g if you want to re-calculate it. 1 mole is equal to 1 moles Water or 1801528 grams. Water molecular weight. 1007942 159994 Percent composition by element.
 Source: slideplayer.com
Source: slideplayer.com
1 mole is equal to 1 moles Hydrogen or 100794 grams. N2g 3 H 2g 2 NH 3g Stoichiometry Chapter 4 Given. 1 mole is equal to 1 moles Water or 1801528 grams. 1 mol N 2 1 molNH 3 719 g NH 3 How many grams of N 2 are required to react completely with 947 grams of H 2. How many grams of ethyl alcohol will result.
 Source: clutchprep.com
Source: clutchprep.com
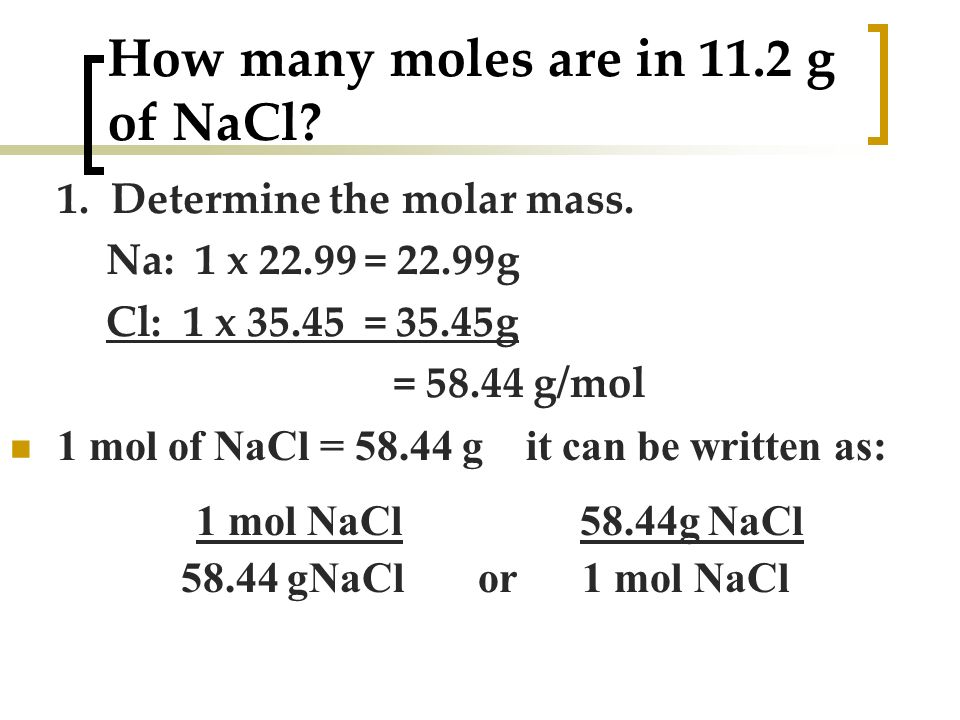
1 mole is equal to 1 moles Fe2O3 or 1596882 grams. 1007942 159994 Percent composition by element. G N 2 Conversion factors. 1 grams Water is equal to 0055508435061792 mole. Did I and do you have to remember them.
 Source: pinterest.com
Source: pinterest.com
Thus one mole of hydrogen has a mass of 1 gram. We assume you are converting between grams Water and mole. How many grams are in 1 mole of Fe2O3. G N 2 Conversion factors. If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol.
 Source: pinterest.com
Source: pinterest.com
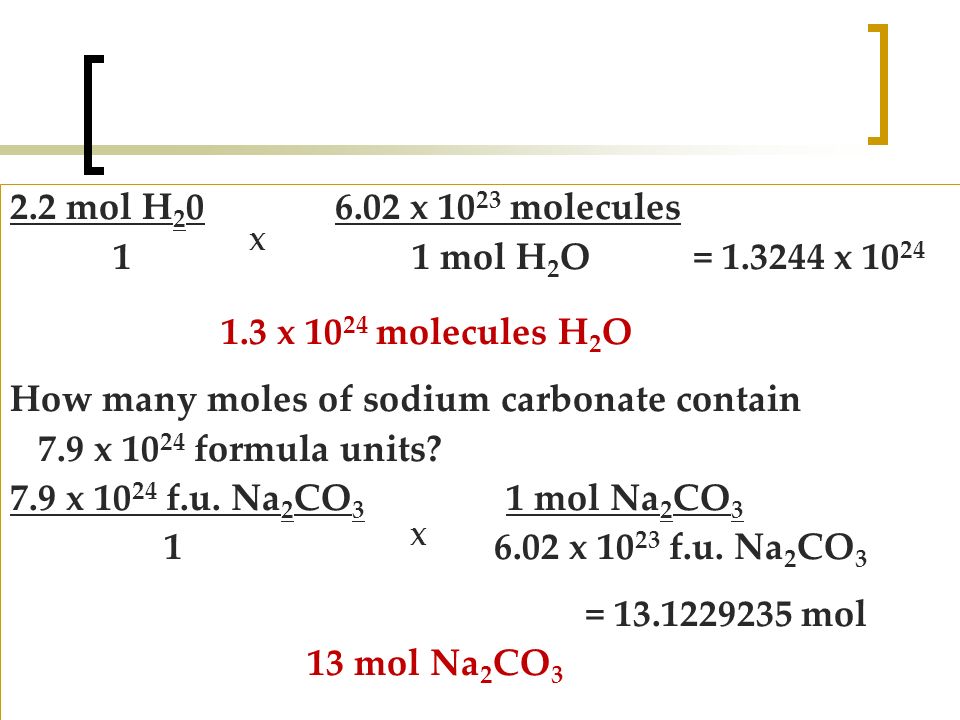
Water molecular weight. I use N A here as I would any other collective number a dozen or a gross or bakers dozen. 00000179 mol HgO X 1 mol O22 mol HgO 00000089 mol O2. 1 mol N 2 1 molNH 3 719 g NH 3 How many grams of N 2 are required to react completely with 947 grams of H 2. Use this page to learn how to convert between moles Water and gram.
 Source: slideplayer.com
Source: slideplayer.com
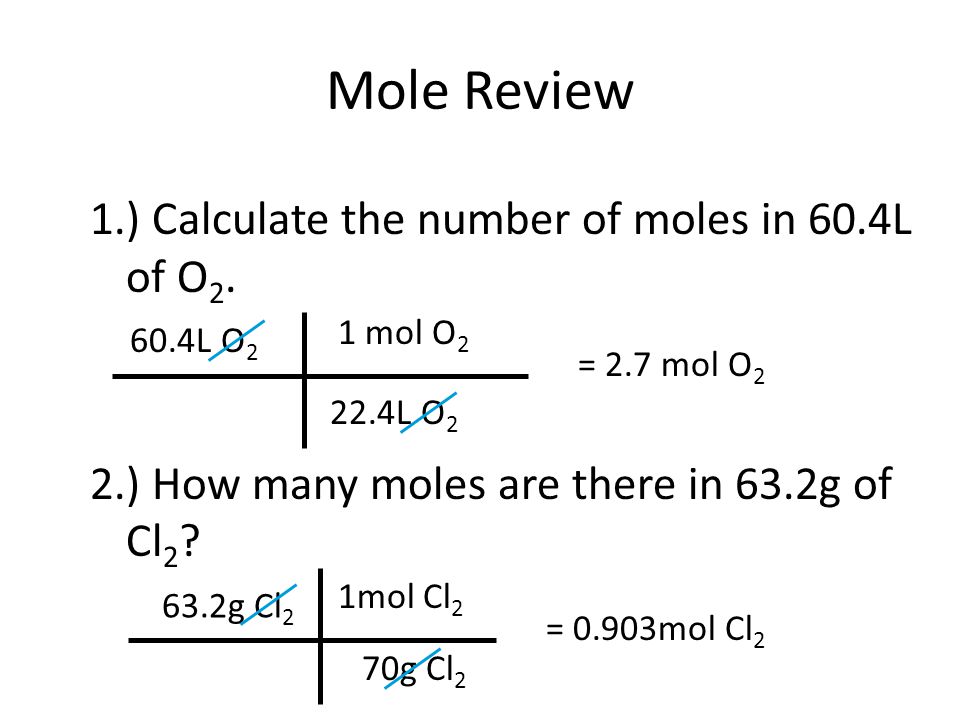
The molar mass of water is 18 g so 100 g of water contains 10018 556 mol. The mass of one mole of a substance is equal to that substances molecular weight. G N 2 Conversion factors. 1007942 159994 Percent composition by element. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
Mass of water H 2 O M 18 g mol 1 Molmass of urea NH 2 CO NH 2 14 2 12 16 14 2 60 g mol 1 According to Raoults law p 0-p p 0 ωM Wm p p 0-w M m W p. Molecular weight of H2O or grams. From where did I get the elemental molar masses. Since we only have 0001 grams that means that we have NA 000118 3351019 molecules of water. I use N A here as I would any other collective number a dozen or a gross or bakers dozen.
 Source: pinterest.com
Source: pinterest.com
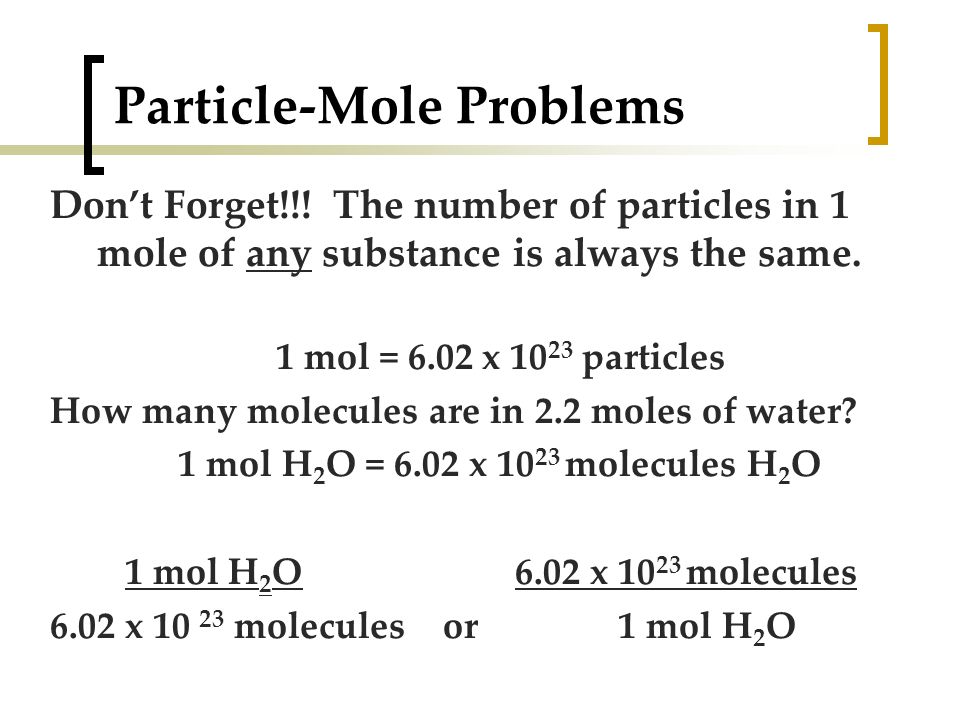
1 mole is equal to 1 moles Water or 1801528 grams. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Water or 1801528 grams. 54 g H2O 1 mol H2O 180152 g H2O 6022 1023 molecules 1 mol H2O. N2g 3 H 2g 2 NH 3g Stoichiometry Chapter 4 Given.
 Source: slideplayer.com
Source: slideplayer.com
0000286 g O2 286 X 10-4 g O2. From there you can go from moles to molecules using 6022 1023 molecules in a mole. 1 mole is equal to 1 moles Hydrogen or 100794 grams. Since the mass ratio between hydrogen to oxygen is 116 and we need 2 hydrogens for each oxygen to create water this reaction needs a 18 ratio in a complete reaction. Mass of water H 2 O M 18 g mol 1 Molmass of urea NH 2 CO NH 2 14 2 12 16 14 2 60 g mol 1 According to Raoults law p 0-p p 0 ωM Wm p p 0-w M m W p.
 Source: pinterest.com
Source: pinterest.com
Molar mass C2H4 2805 gmol 0130mol 01302805 365g Your answer correct. Vapoure pressure of pure water solvent at 298 K p 0 238 mm Vapour pressure of solution p. Avogadros number is the conversion factor from atomic mass units AMU to grams 1 gram 602 1023 AMU. Fortunately this is another simple calculation. The mass of one mole of a substance equals its relative molecular mass expressed in grams.
 Source: pinterest.com
Source: pinterest.com
651g presume 0361 mol. 1 mole is equal to 1 moles H2O or 1801528 grams. How many grams of ethyl alcohol will result. Molar mass of H2O 1801528 gmol. 1 mole is equal to 1 moles Water or 1801528 grams.
 Source: slideplayer.com
Source: slideplayer.com
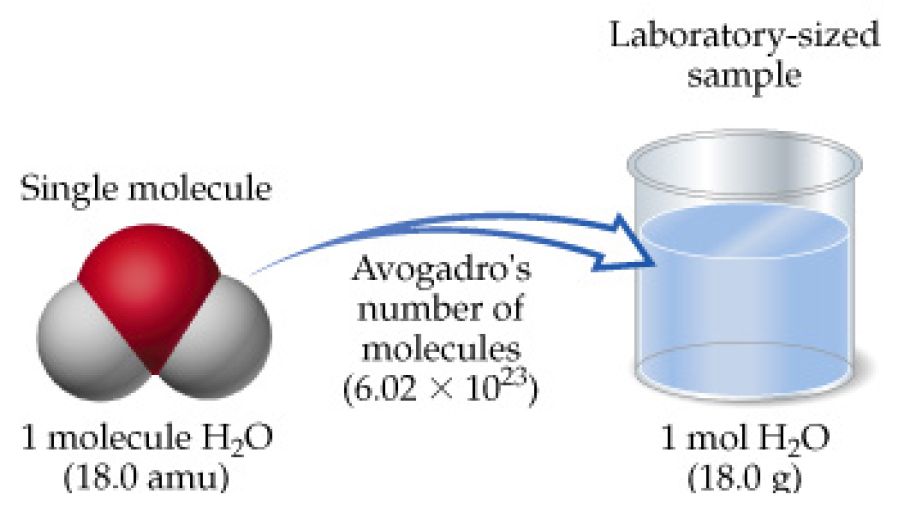
0000286 g O2 286 X 10-4 g O2. This compound is also known as Water or Dihydrogen Monoxide. A T chart is useful for this kind of problem. One mole of water H2O has a mass of 18 grams. I use N A here as I would any other collective number a dozen or a gross or bakers dozen.
 Source: socratic.org
Source: socratic.org
Atomic Mass of water is 2x Hydrogen 1 x Oxygen. The SI base unit for amount of substance is the mole. The answer is 1801528. Its easier to grasp how much water is in a mole if you find the volume of this amount of mass. Therefore one mole of water weighs 180152 grams.
 Source: slideplayer.com
Source: slideplayer.com
Fortunately this is another simple calculation. Molecular weight of H2O or grams. Use this page to learn how to. Since the mass ratio between hydrogen to oxygen is 116 and we need 2 hydrogens for each oxygen to create water this reaction needs a 18 ratio in a complete reaction. Did I and do you have to remember them.
 Source: pinterest.com
Source: pinterest.com
We assume you are converting between grams Water and mole. 1007942 159994 Percent composition by element. So in 0050 g water there are 0050 g 18015 g mol1 N A 28 103 N A water molecules. The answer is 1801528. Molar mass of H2O 1801528 gmol.
 Source: pinterest.com
Source: pinterest.com
For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. From there you can go from moles to molecules using 6022 1023 molecules in a mole. Weight of water 180152 g. Atomic Mass of water is 2x Hydrogen 1 x Oxygen. The molar mass of water is 18 g so 100 g of water contains 10018 556 mol.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is 1 mol of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






