Your How many grams is 1 mol of nh3 images are ready. How many grams is 1 mol of nh3 are a topic that is being searched for and liked by netizens today. You can Get the How many grams is 1 mol of nh3 files here. Find and Download all royalty-free photos.
If you’re searching for how many grams is 1 mol of nh3 pictures information linked to the how many grams is 1 mol of nh3 keyword, you have pay a visit to the ideal site. Our website frequently provides you with suggestions for viewing the highest quality video and picture content, please kindly search and find more informative video content and images that match your interests.
How Many Grams Is 1 Mol Of Nh3. This means 17 g of ammonia gas is equivalent to 1 mole of ammonia gas molecule. 250 g N21 mol N22802 g N22 mol NO1 mol N23001 g NO1 mol NO 5355 g NO b. Calculate the moles of NH3. Molecular weight of NH3 or grams This compound is also known as Ammonia.
 Ch Stoichiometry N2 G H2 G Nh3 G Ppt Download From slideplayer.com
Ch Stoichiometry N2 G H2 G Nh3 G Ppt Download From slideplayer.com
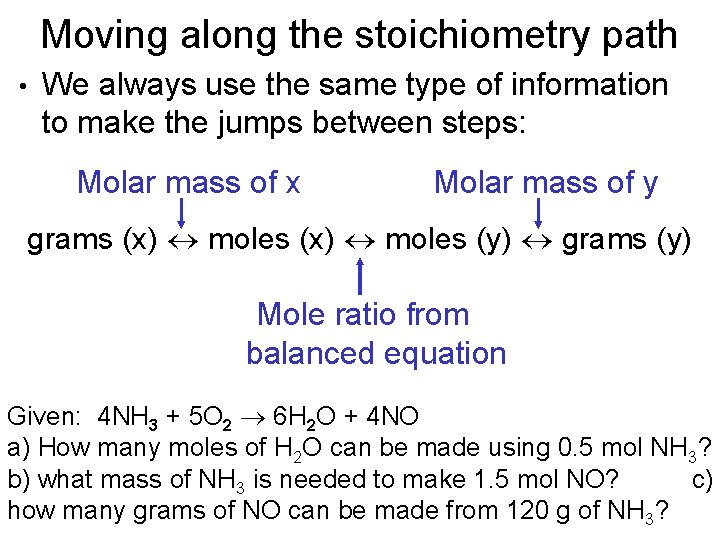
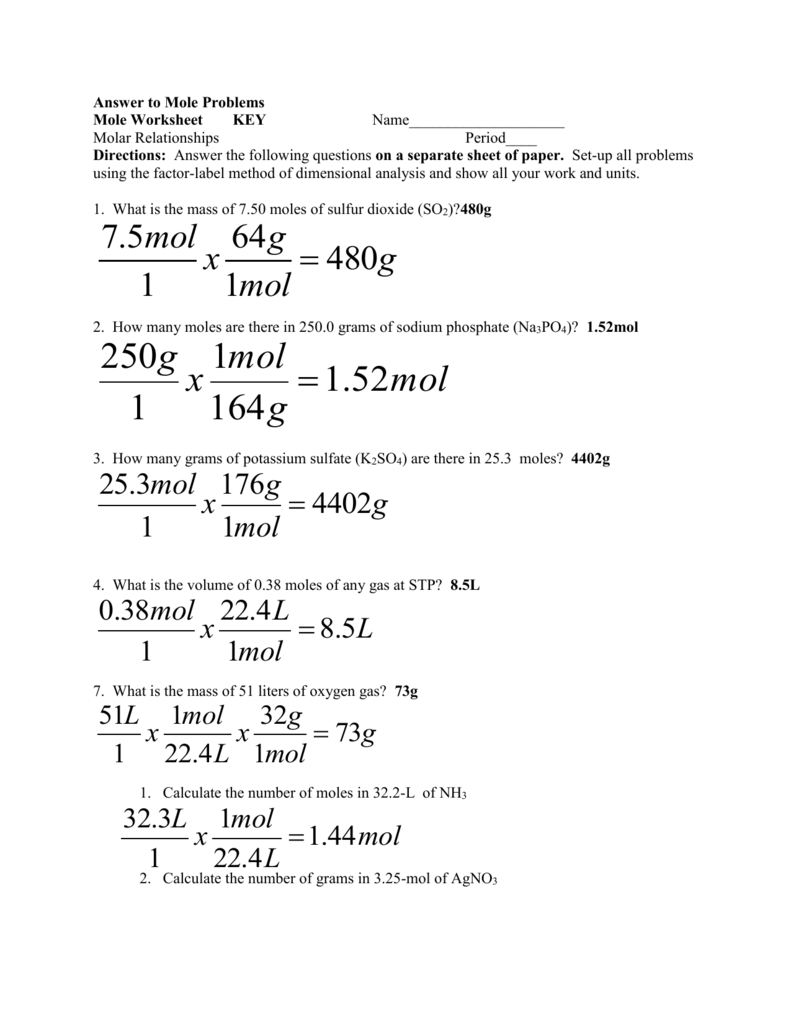
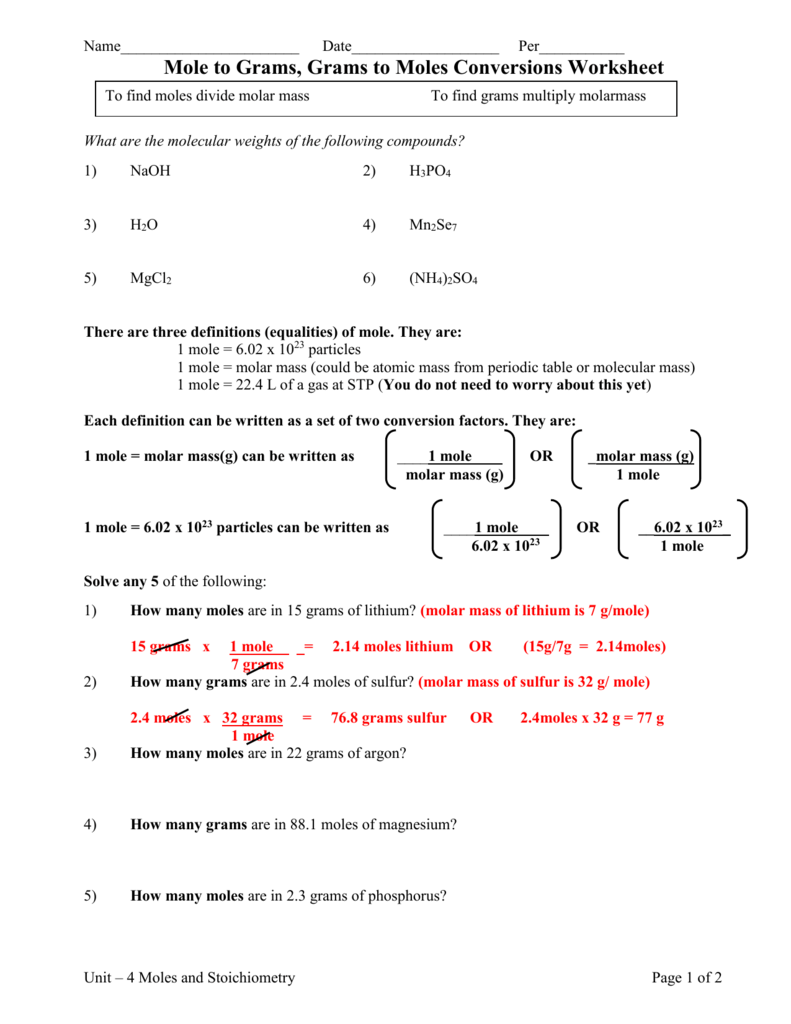
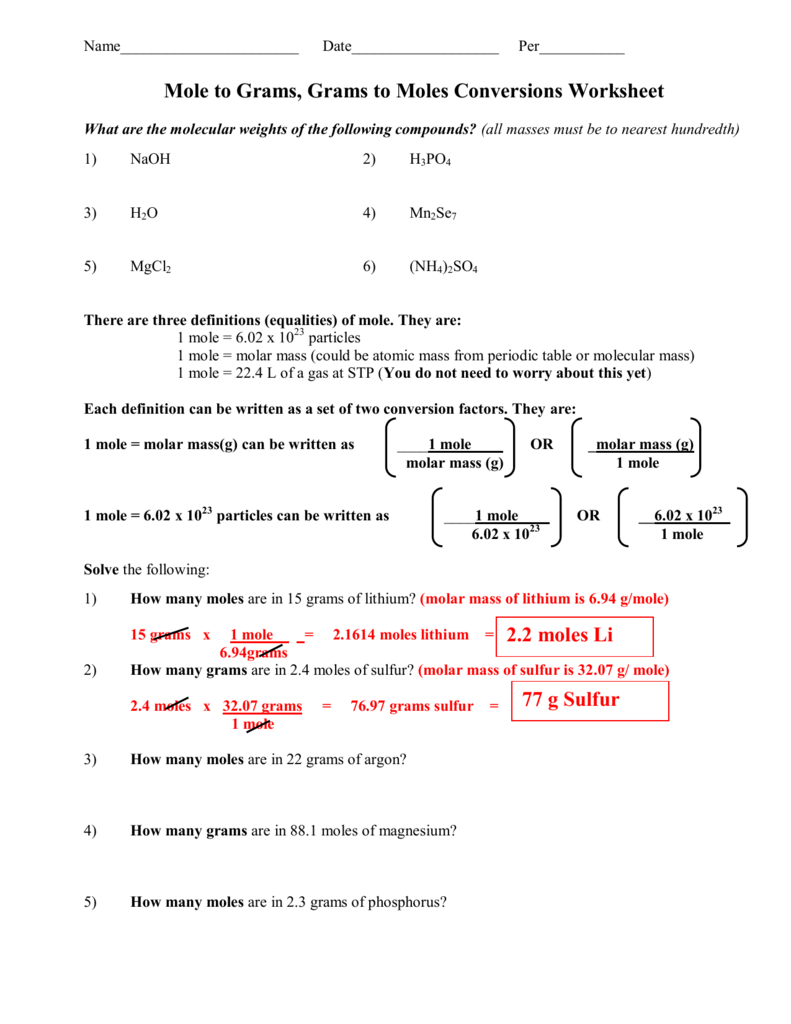
Molar Mass of H2 202 grams Molar Mass of NH3 1704 grams 1 mol of NO 3001 grams 1 mol of H2 202 grams 1 mol of NH3 1704 grams. 16810 24 atoms of nitrogen are present in 279 mol of NH 3. 1 Answer 01 mole Something went wrong. 250 g N21 mol N22802 g N22 mol NO1 mol N23001 g NO1 mol NO 5355 g NO b. Molar mass of ammonia NH3 14 13 14 3 17 gmol Mass of ammonia 170 g No. Mass of NH3 488mol NH3 1703 g NH3 1mol NH3 831 g NH3.
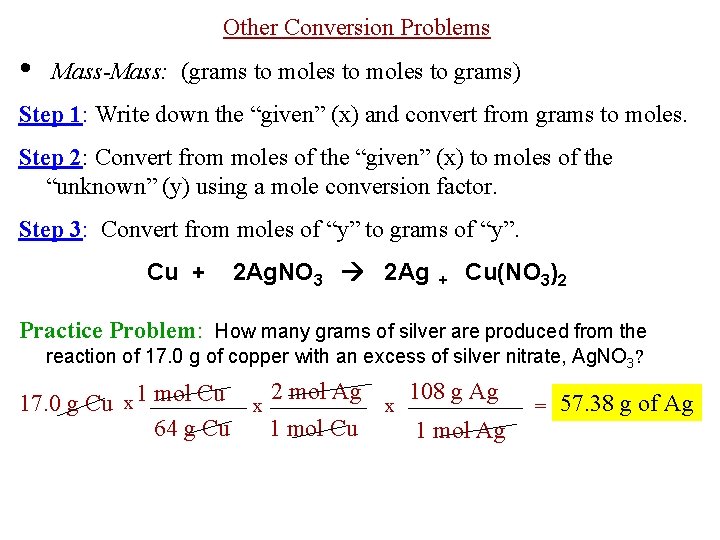
That 2 moles of NH3 combined with 1 mole of H2SO4 produces one mole of NH42SO4 So for every mole of H2SO4 that reacts we produce one mole of NH42SO4.
M is the molar mass of the substance the mass of one mole of the substance in g mol-1. B Mass of H2. This means 17 g of ammonia gas is equivalent to 1 mole of ammonia gas molecule. The SI base unit for amount of substance is the mole. How many moles of nitrogen are in one mole of NH3. The definition of Avogadros number of 6022 1023mole is the number of atoms or molecules per one gram atomic weight.
 Source: pinterest.com
Source: pinterest.com
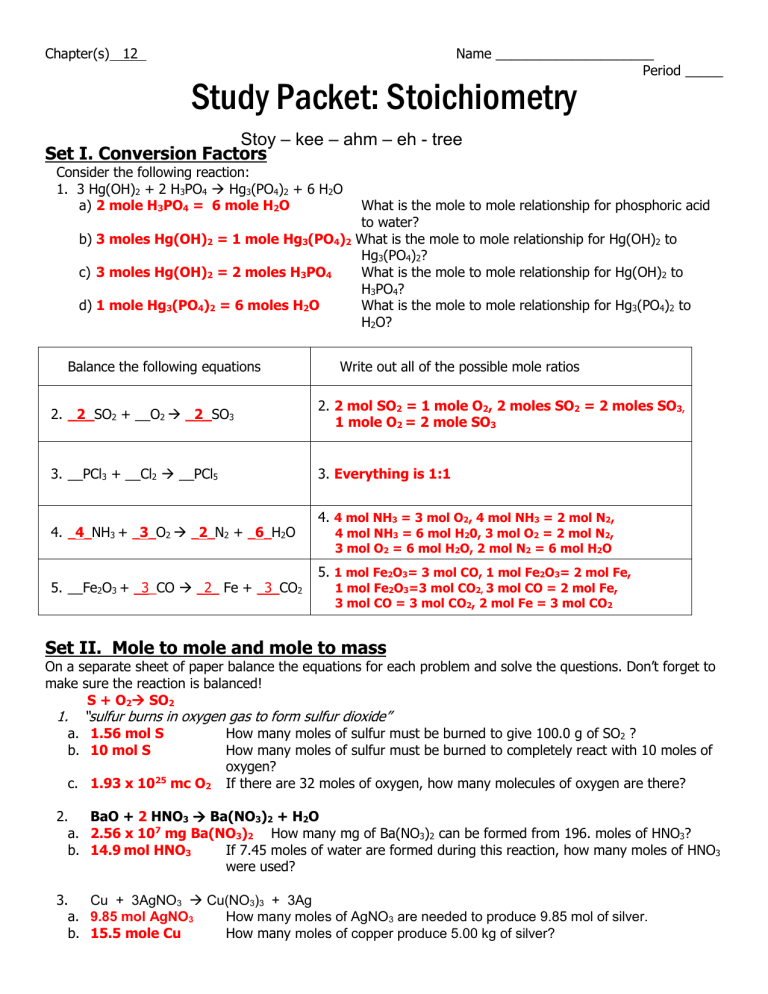
1mol of nitrogen has a mass of 2802g while 3mol of hydrogen has a mass of 606g and 2mol of ammonia has a mass of 3408g. 1 mole is equal to 1 moles NH3 or 1703052 grams. One mole of NH3 weighs 17. Note that rounding errors may occur so always check the results. NH3 has an atomic mass of 17.
 Source: slidetodoc.com
Source: slidetodoc.com
You can view more details on each measurement unit. Calculate the moles of NH3. The number of molecules in 34 g NH3 is shown below. 250 g N21 mol N22802 g N22 mol NO1 mol N23001 g NO1 mol NO 5355 g NO b. 1 mole is equal to 1 moles NH3 or 1703052 grams.
 Source: studylib.net
Source: studylib.net
Indicate which is the limiting reactantN2 3H2 2NH3My answer250g N2 x 1 mol 280gN2 x 2 mol. 1 Expert Answer molecules NH3 147 moles x 6021023 moleculesmole 891023 molecules of NH3 to 2 sig. Molecular weight of NH3 or grams This compound is also known as Ammonia. The SI base unit for amount of substance is the mole. The definition of Avogadros number of 6022 1023mole is the number of atoms or molecules per one gram atomic weight.
 Source: studylib.net
Source: studylib.net
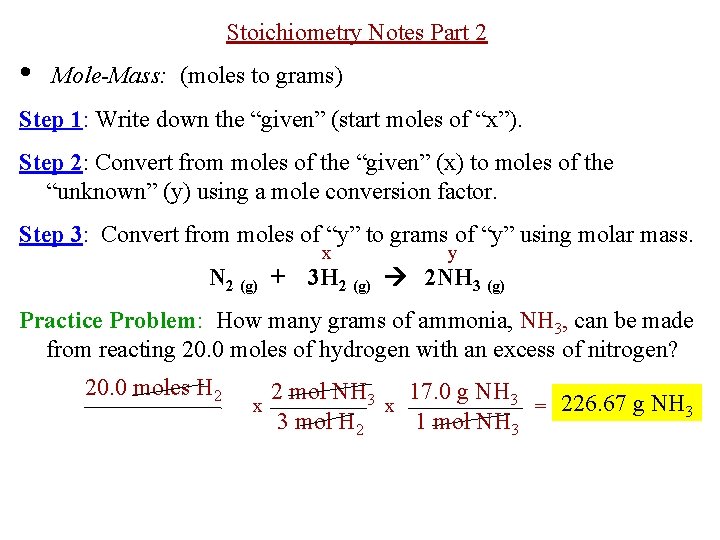
The Avagadros number is the unit in one mole of any substance also stated as molecular weight in grams equal to 60221023. So in one mole of nitrogen gas you have 60221023 molecules of nitrogen gas N2. M is the mass of the substance in grams g. Molar Mass of H2 202 grams Molar Mass of NH3 1704 grams 1 mol of NO 3001 grams 1 mol of H2 202 grams 1 mol of NH3 1704 grams. Moles of NH3 244mol N2 2 mol NH3 1mol N2 488lmol NH3.
 Source: slideplayer.com
Source: slideplayer.com
1 Answer 01 mole Something went wrong. 250 g N21 mol N22802 g N22 mol NO1 mol N23001 g NO1 mol NO 5355 g NO b. Since molar mass of NH3 is 17 1413 one mole contains 17 gramHence 9 moles are present in 153gm153179. 1 mole of nitrogen has a mass of 2802 g while 3 moles of hydrogen have a mass of 606 g and 2 moles of ammonia have a mass of 3408 g. Let us first calculate the molar mass of ammonia gas.
 Source: youtube.com
Source: youtube.com
We assume you are converting between moles NH3 and gram. Nitrogen has an atomic mass of 14. 250 g N21 mol N22802 g N22 mol NO1 mol N23001 g NO1 mol NO 5355 g NO b. M is the mass of the substance in grams g. 1 mole is equal to 1 moles NH3 or 1703052 grams.
 Source: slideplayer.com
Source: slideplayer.com
The answer is 1703052. Molar Mass of H2 202 grams Molar Mass of NH3 1704 grams 1 mol of NO 3001 grams 1 mol of H2 202 grams 1 mol of NH3 1704 grams. The molecular weight of NH3 is 1703-grams per mole and 1401 for N2. Answer 1 of 3. M is the molar mass of the substance the mass of one mole of the substance in g mol-1.
 Source: studylib.net
Source: studylib.net
The SI base unit for amount of substance is the mole. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles NH3 or 1703052 grams. 4 NH3 5 O2 6 H2O 4 NO a b c Answers 6 mol H2O 4 mol NH3 x16 mol NH3 24 mol H2O 4 mol NH3 4 mol NO x075 mol NO 13 g NH3 1704 g NH3 1 mol NH3 x 4 mol NO 4 mol NH3 x47 g NH3 83 g NO 3001 g NO 1 mol NO x 1 mol NH3 1704gNH3 x. One mole of any substance contains 602210 23 atomsmolecules.
 Source: slidetodoc.com
Source: slidetodoc.com
Calculate the mass of NH3. Wait a moment and try again. So it is a 11 one-to-one ratio of NH3 and NH42SO4 So we can similarly conclude that it takes 60 moles of NH3 to produce 60 moles of NH42SO4. We assume you are converting between moles NH3 and gram. 1 grams NH3 is equal to 0058718113128665 mole.
 Source: studylib.net
Source: studylib.net
60 moles of NH4. Hydrogen has an atomic mass of 1. One mole of NH3 weighs 17. Note that rounding errors may occur so always check the results. The weight of the 02 mol atoms is 16 02 which corresponds to 32 grams.
 Source: slideplayer.com
Source: slideplayer.com
Since molar mass of NH3 is 17 1413 one mole contains 17 gramHence 9 moles are present in 153gm153179. Use this page to learn how to convert between moles NH3 and gram. Indicate which is the limiting reactantN2 3H2 2NH3My answer250g N2 x 1 mol 280gN2 x 2 mol. Answer and Explanation. The Avagadros number is the unit in one mole of any substance also stated as molecular weight in grams equal to 60221023.
 Source: studylib.net
Source: studylib.net
Mass of NH3 488mol NH3 1703 g NH3 1mol NH3 831 g NH3. We assume you are converting between grams NH3 and mole. Moles of NH3 244mol N2 2 mol NH3 1mol N2 488lmol NH3. Molar mass molar ratio g N 2 947 g H 2 x 1 mol H 2 x 1 mol N 2 x 2801g N 2 2016 g H 2 3 mol H 2 1 molN2 439 g N 2 Stoichiometry. 1 mole is equal to 1 moles NH3 or 1703052 grams.
 Source: youtube.com
Source: youtube.com
N mM n is the amount of substance in moles mol. 1 Answer 01 mole Something went wrong. Molecular weight of NH3 or grams This compound is also known as Ammonia. 1 mole is equal to 1 moles NH3 or 1703052 grams. How many molecules are present in NH3.

How many grams are in 2 moles of NH3. Moles of NH3 1275g NH3 1 mol NH3 1703g NH3 0748 68 mol NH3. Answer and Explanation. Molar Mass of H2 202 grams Molar Mass of NH3 1704 grams 1 mol of NO 3001 grams 1 mol of H2 202 grams 1 mol of NH3 1704 grams. That 2 moles of NH3 combined with 1 mole of H2SO4 produces one mole of NH42SO4 So for every mole of H2SO4 that reacts we produce one mole of NH42SO4.
 Source: slidetodoc.com
Source: slidetodoc.com
Wait a moment and try again. Even though there is enough N2 to produce more NO there is not enough O2 to react with all the N2 available. This means 17 g of ammonia gas is equivalent to 1 mole of ammonia gas molecule. How many molecules are present in NH3. G N 2 Conversion factors.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is 1 mol of nh3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






