Your How many grams is 1 mol of naoh images are available. How many grams is 1 mol of naoh are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams is 1 mol of naoh files here. Find and Download all royalty-free photos.
If you’re searching for how many grams is 1 mol of naoh images information connected with to the how many grams is 1 mol of naoh keyword, you have visit the right blog. Our website always provides you with hints for seeing the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and images that fit your interests.
How Many Grams Is 1 Mol Of Naoh. Molarity variety of moles of naoh quantity of resolution in litre. How many grams of magnesium metal will react completely with 83 liters of 55 m hcl. Molecular weight of NaOH or mol This compound is also known as Sodium Hydroxide. Molar mass of NaOH 3999711 gmol.
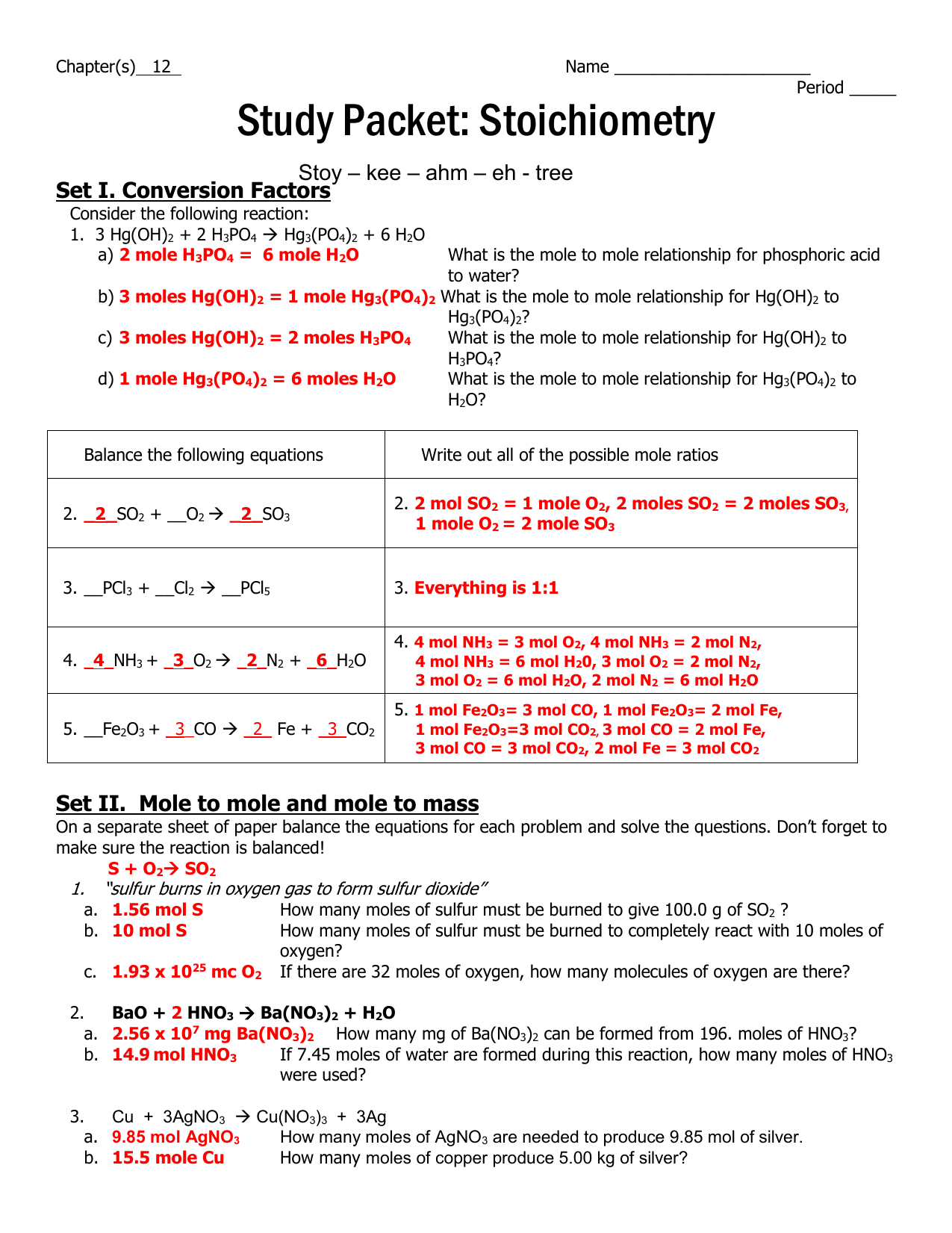 Chapter S 12 Name Period Study Packet Stoichiometry Set I From studylib.net
Chapter S 12 Name Period Study Packet Stoichiometry Set I From studylib.net
Sodium oxygen and hydrogen. 2298977 159994 100794 Percent composition by element. Add all of these up to get the molar mass of NaOH is 40 gmol. 1 mole is equal to 1 moles NaOH or 3999711 grams. Show all of the work needed to solve this problem. How many grams of NaOH are contained in 024 mole of NaOH.
How many grams of magnesium metal will react completely with 83 liters of 55 m hcl.
Mg s 2hcl aq mgcl2 aq h2 g. Use this page to learn how to convert between moles NaOH and gram. The answer is 3999711. Molecular weight of Sodium Hydroxide or grams. Show all of the work needed to solve this problem. Calculate the moles of ammonia present in a 9406 g sample if the molar mass of ammonia is 17030 g.
 Source: slideplayer.com
Source: slideplayer.com
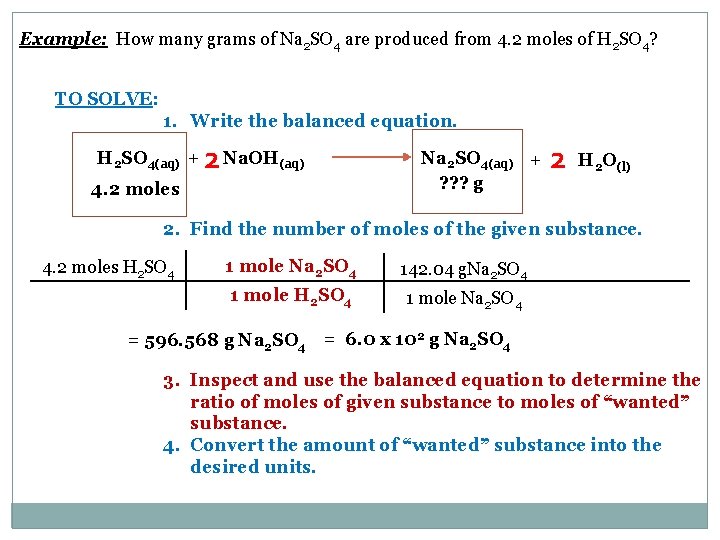
The gfm of NaOH is 1 x 23 1 x 16 1 x 1 40 The mole ratio from the balanced chemical equation is 2 moles of NaOH for every mole mole of Na_2O. Mass of NaOH g 0400 volume of NaOH solution mL 20000 mass of benzoic acid g 0158 volume of benzoic acid solution mL 10000 volume of NaOH dispensed mL 2784. One thousandth of this is 4 grams giving 1 mM. Molarity variety of moles of naoh quantity of resolution in litre. The SI base unit for amount of substance is the mole.
 Source: slidetodoc.com
Source: slidetodoc.com
The gfm of NaOH is 1 x 23 1 x 16 1 x 1 40 The mole ratio from the balanced chemical equation is 2 moles of NaOH for every mole mole of Na_2O. Molar mass NaOH 400 gmol Mass of 15 mol NaOH 40 gmol 5 mol 60 g NaOH 15 M solution NaOH 60 g NaOH in 10 L solution. Molar mass of NaOH 3999711 gmol. The final calculation is 120 x 2 x 40 62 1548387. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of Sodium Hydroxide or mol The molecular formula for Sodium Hydroxide is NaOH. Use this page to learn how to convert between moles NaOH and gram. The compound sodium hydroxide NaOH comprises the atoms. For our practice problem well. 3 Show answers Another question on Chemistry.
 Source: pinterest.com
Source: pinterest.com
How many grams are in one mole of salt. The final calculation is 120 x 2 x 40 62 1548387. Since the molar mass of NaOH is 40gmol a 1 M solution of NaOH would be 40 g 1 mole of NaOH in 1 liter. Molar mass NaOH 400 gmol Mass of 15 mol NaOH 40 gmol 5 mol 60 g NaOH 15 M solution NaOH 60 g NaOH in 10 L solution. Calculate the moles of ammonia present in a 9406 g sample if the molar mass of ammonia is 17030 g.
 Source: studylib.net
Source: studylib.net
The final calculation is 120 x 2 x 40 62 1548387. How many grams are in one mole of salt. How many grams of NaOH are contained in 024 mole of NaOH. If playback doesnt begin shortly try restarting your device. Use this page to learn how to convert between moles NaOH and gram.
 Source: pinterest.com
Source: pinterest.com
In this video well learn to convert moles of NaOH to grams. Since the molar mass of NaOH is 40gmol a 1 M solution of NaOH would be 40 g 1 mole of NaOH in 1 liter. Molecular weight of Sodium Hydroxide or mol The molecular formula for Sodium Hydroxide is NaOH. 1 mole is the same as 1 moles NaOH or 3999711 grams. If playback doesnt begin shortly try restarting your device.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of Sodium Hydroxide or mol The molecular formula for Sodium Hydroxide is NaOH. 1 Mole Na 0357 Moles Na 230 g Na Step 2. Sodium oxygen and hydrogen. Molar mass NaOH 400 gmol Mass of 15 mol NaOH 40 gmol 5 mol 60 g NaOH 15 M solution NaOH 60 g NaOH in 10 L solution. The SI base unit for amount of substance is the mole.
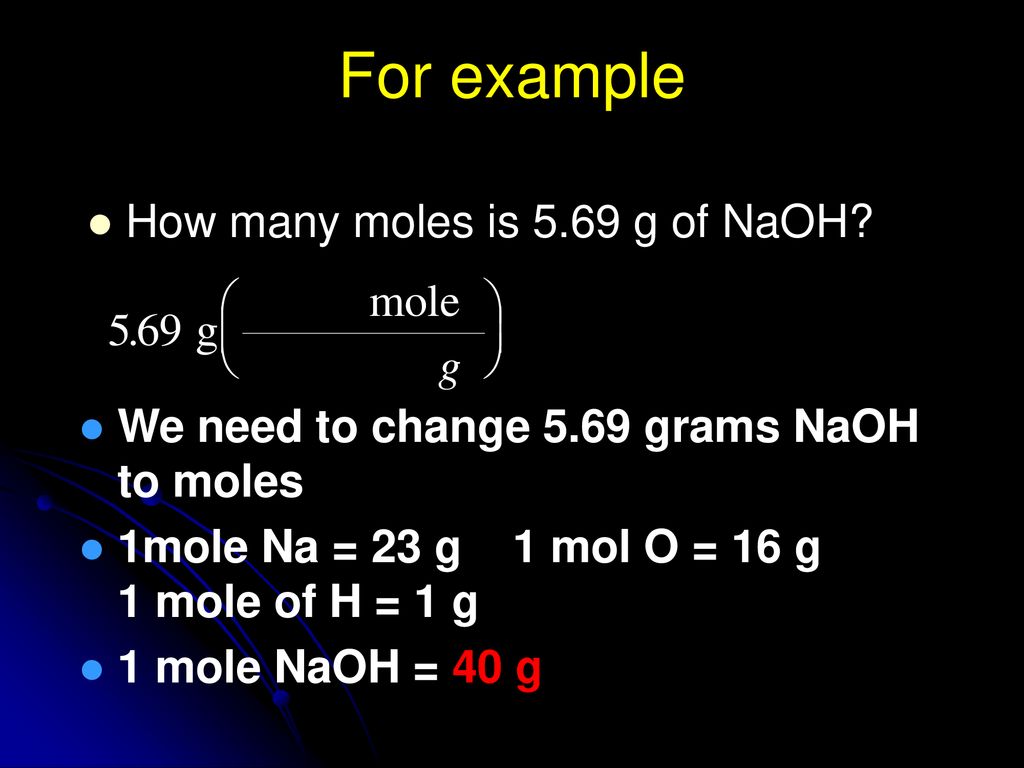 Source: slideplayer.com
Source: slideplayer.com
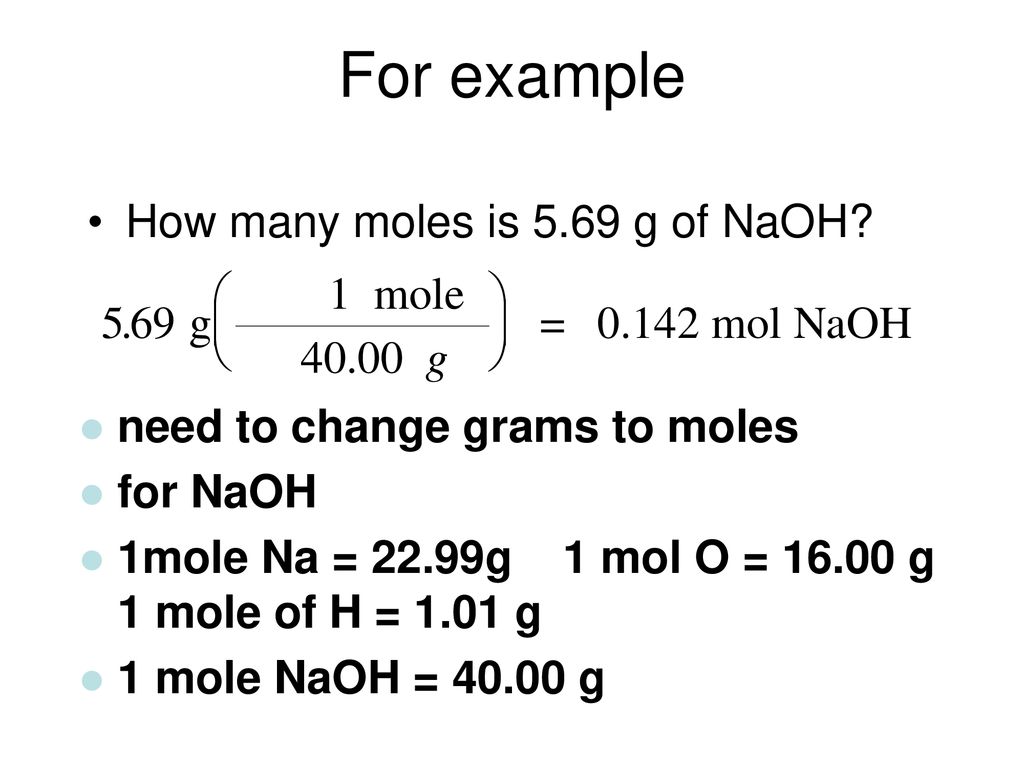
2298977 159994 100794 Percent composition by element. 00513 mol 400 g mol1. Now take 120 grams NaOh and multiply this by 1 mol NaOH 40 grams NaOH. For our practice problem well. Molecular weight of Sodium Hydroxide or grams.
 Source: slideplayer.com
Source: slideplayer.com
Since the molar mass of NaOH is 40gmol a 1 M solution of NaOH would be 40 g 1 mole of NaOH in 1 liter. How many grams Sodium Hydroxide in 1 mol. Since the molar mass of NaOH is 40gmol a 1 M solution of NaOH would be 40 g 1 mole of NaOH in 1 liter. Now use this to convert 120 g to moles. 1 mole is equal to 1 moles NaOH or 3999711 grams.
 Source: slideplayer.com
Source: slideplayer.com
Calculate the moles of ammonia present in a 9406 g sample if the molar mass of ammonia is 17030 g. The answer is 3999711. 2298977 159994 100794 Percent composition by element. The SI base unit for amount of substance is the mole. Molarity is outlined as moles of solute which in your case is sodium hydroxide naoh.
 Source: studylib.net
Source: studylib.net
The answer is 3999711. Molar Mass of Ammonia 17030 gmol Mass of Ammonia 9406g To Find. What is the exact concentration of the NaOH solution. The compound sodium hydroxide NaOH comprises the atoms. Add all of these up to get the molar mass of NaOH is 40 gmol.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. Molecular weight of Sodium Hydroxide or mol The molecular formula for Sodium Hydroxide is NaOH. Mg s 2hcl aq mgcl2 aq h2 g. Molecular weight of Sodium Hydroxide or grams. You can view more details on each measurement unit.
 Source: in.pinterest.com
Source: in.pinterest.com
The final solution comes to 154. In this video well learn to convert moles of NaOH to grams. Molarity is outlined as moles of solute which in your case is sodium hydroxide naoh. Sodium oxygen and hydrogen. What is the exact concentration of the NaOH solution.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles NaOH and gram. Mg s 2hcl aq mgcl2 aq h2 g. Molarity is outlined as moles of solute which in your case is sodium hydroxide naoh. Na mass is 23 O has 16 and H is 1. Molecular weight of Sodium Hydroxide or mol The molecular formula for Sodium Hydroxide is NaOH.

Na mass is 23 O has 16 and H is 1. 1 mole is equal to 1 moles NaOH or 3999711 grams. Answer 1 of 2. Now use this to convert 120 g to moles. Now take 120 grams NaOh and multiply this by 1 mol NaOH 40 grams NaOH.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is 1 mol of naoh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






