Your How many grams is 1 mol of h2o images are available in this site. How many grams is 1 mol of h2o are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams is 1 mol of h2o files here. Find and Download all free photos and vectors.
If you’re looking for how many grams is 1 mol of h2o pictures information linked to the how many grams is 1 mol of h2o interest, you have pay a visit to the right blog. Our site frequently gives you suggestions for seeking the maximum quality video and image content, please kindly hunt and find more informative video articles and images that match your interests.
How Many Grams Is 1 Mol Of H2o. On StudySoup on 5312017. 1 mole is equal to 1 moles H2O or 1801528 grams. D the number of atoms of each element is the same in reactants and products. The technique used can be applied to any mole to gram conversion.
 Grams To Moles Water Example Youtube From youtube.com
Grams To Moles Water Example Youtube From youtube.com
The molar mass of water is 1802 gmol. Use this page to learn how to convert between moles H2O and gram. In this video well learn to convert moles of H2O to grams. A the total number of molecules is the same in reactants and products. Na2O H2O 2 NaOH. So in 18 g of water there will be.
Use dimensional analysis and you will get about 3186 g.
Since water has a chemical formula of H 2O there will be 2 moles of hydrogen in every mole of water. One mole of water H2O has a mass of 18 grams. 18g 18gmol 1 mol. CaSO42H2O Heat CaSO412H2O 32 H2O - So 32 moles of water leaves from it. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. 0000286 g O2 286 X 10-4 g O2.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. Thus one mole of hydrogen has a mass of 1 gram. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. Use this page to learn how to convert between moles H2O and gram. Note that rounding errors may occur so always check the results.

Molecular weight of H2O ormol This compound is also known as Water or Dihydrogen Monoxide. On StudySoup on 5312017. The molecular weights of H 2 O 2 and H 2 O are 2 gmol 32 gmol and 18 gmole respectively. Mr of water is 1611 18 gmol. The number of atoms is an exact number the number of mole is an exact number.
 Source: amburnclasses.org
Source: amburnclasses.org
Molecular weight of Water or mol The molecular formula for Water is H2O. The technique used can be applied to any mole to gram conversion. So in 18 g of water there will be. 26 780 g CH 26 1 mol CH x 26 3006 g CH 2 6 mol HO x 26 2 mol CH 2 2 180 g HO x 1 mol HO 2 140. Molecular weight of Water or mol The molecular formula for Water is H2O.
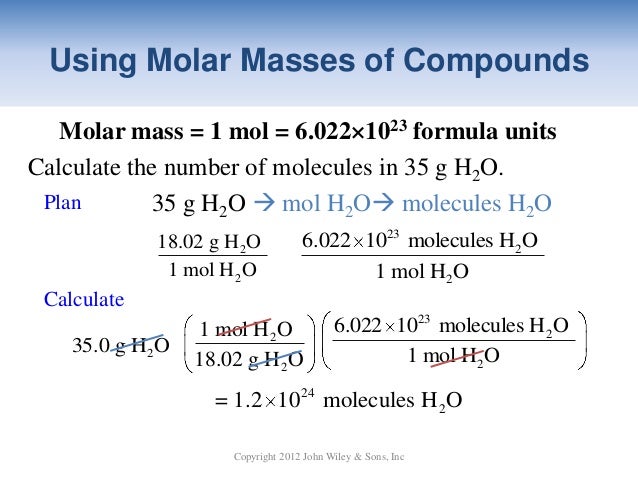 Source: slideshare.net
Source: slideshare.net
If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol. Note that rounding errors may occur so always check the results. Water has a molar mass of 1801528 gmol 18 gmol. Molar mass is a convenient means of keeping track of the masses of various compounds involved in chemical reactions. G H 2O from H 2 100 g H 2 x 1 mol H 2 x 2 mol H 2O 202 g H 2 2 mol H 2 x 180 g H 2O 891 g H 2O 1 mol H 2O Limiting Reactants Chapter 4 g H2O from O 2 750 g O 2 x 1 mol O 2 x 2 mol H 2O 320 g O 2 1 mol O 2 x 180 g H 2O 844 g H 2O 1 mol H 2O Limiting Reactants Chapter 4 Limiting reagent is the one that produces the least product.
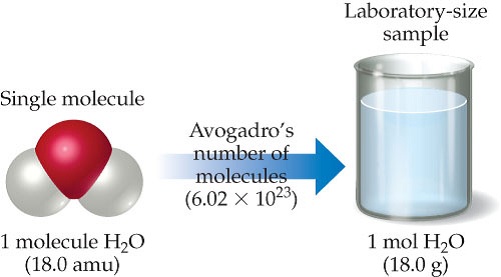 Source: chegg.com
Source: chegg.com
Use dimensional analysis and you will get about 3186 g. - The molar mass of water 18 gm Mass of 32 moles 18 32 27 gm - So 27 grams of mass decrease is observed on converting 1 mole of gypsum into 1 mole of. The number of atoms is an exact number the number of mole is an exact number. The SI base unit for amount of substance is the mole. C the sum of the coefficients of the reactants is equal to the sum of the coefficients of the products.
 Source: slideplayer.com
Source: slideplayer.com
Water has a molar mass of 1801528 gmol 18 gmol. How many grams of NaOH 4000 gmol is produced from 120 grams of Na2O 6198 gmol. Use dimensional analysis and you will get about 3186 g. The SI base unit for amount of substance is the mole. The molar mass of water is 1802 gmol.
 Source: youtube.com
Source: youtube.com
Avogadros number is the conversion factor from atomic mass units AMU to grams 1 gram 602 1023 AMU. In this video well learn to convert moles of H2O to grams. 1 mol O x 2 320 g O 2 2 4 mol CO x 7 mol O 2 0446 mol CO 10. 120 g Na2O x 1 mol Na2O x 2 mol NaOH x 4000 g NaOH 155 g NaOH. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g.
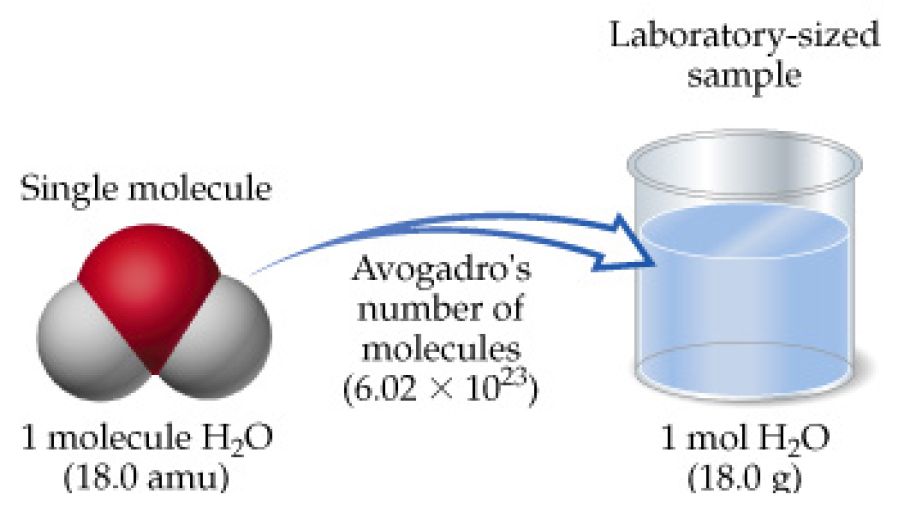 Source: socratic.org
Source: socratic.org
Molecular weight of H2O ormol This compound is also known as Water or Dihydrogen Monoxide. They do not affect the number of significant figures. Use this page to learn how to convert between moles H2O and gram. The technique used can be applied to any mole to gram conversion. Molecular weight of Water or grams The molecular formula for Water is H2O.
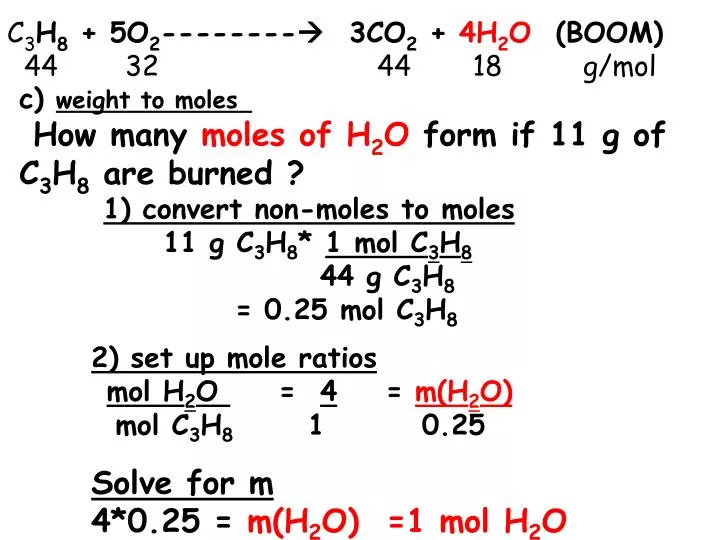 Source: slideserve.com
Source: slideserve.com
The question contains content related to Chemistry and Science. We assume you are converting between grams H2O and mole. In this video well learn to convert moles of H2O to grams. Note that rounding errors may occur so always check the results. 2 g 1 mole of H 2 combines with 16 g half mole of O 2 to form 18 g one mole of H 2 O.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of Water or mol The molecular formula for Water is H2O. 26 780 g CH 26 1 mol CH x 26 3006 g CH 2 6 mol HO x 26 2 mol CH 2 2 180 g HO x 1 mol HO 2 140. On StudySoup on 5312017. A the total number of molecules is the same in reactants and products. M 130 x 18.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between moles Water and gram. We assume you are converting between grams Water and mole. Na2O H2O 2 NaOH. Given the following equation. 26 780 g CH 26 1 mol CH x 26 3006 g CH 2 6 mol HO x 26 2 mol CH 2 2 180 g HO x 1 mol HO 2 140.
 Source: slideserve.com
Source: slideserve.com
Water has a molar mass of 1801528 gmol 18 gmol. 1 grams H2O is equal to 0. - The formula of Plaster of Paris is CaSO412H2O. Since water has a chemical formula of H 2O there will be 2 moles of hydrogen in every mole of water. D the number of atoms of each element is the same in reactants and products.
 Source: sites.google.com
Source: sites.google.com
How many grams Water in 1 mol. The SI base unit for amount of substance is the mole. 26 780 g CH 26 1 mol CH x 26 3006 g CH 2 6 mol HO x 26 2 mol CH 2 2 180 g HO x 1 mol HO 2 140. If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol. 1 mole is equal to 1 moles H2O or 1801528 grams.
 Source: slideplayer.com
Source: slideplayer.com
C the sum of the coefficients of the reactants is equal to the sum of the coefficients of the products. The question contains content related to Chemistry and Science. Avogadros number is the conversion factor from atomic mass units AMU to grams 1 gram 602 1023 AMU. The molecular weights of H 2 O 2 and H 2 O are 2 gmol 32 gmol and 18 gmole respectively. The average mass of one mole of H2O is 1802 grams.
 Source: youtube.com
Source: youtube.com
C the sum of the coefficients of the reactants is equal to the sum of the coefficients of the products. 1 mol O x 2 320 g O 2 2 4 mol CO x 7 mol O 2 0446 mol CO 10. If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol. Note that rounding errors may occur so always check the results. Use this page to learn how to convert between moles Water and gram.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is 1 mol of h2o by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






