Your How many grams is 1 mol of h20 images are available in this site. How many grams is 1 mol of h20 are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams is 1 mol of h20 files here. Download all royalty-free photos and vectors.
If you’re looking for how many grams is 1 mol of h20 images information connected with to the how many grams is 1 mol of h20 topic, you have pay a visit to the ideal blog. Our site always provides you with hints for refferencing the highest quality video and picture content, please kindly surf and find more enlightening video articles and images that fit your interests.
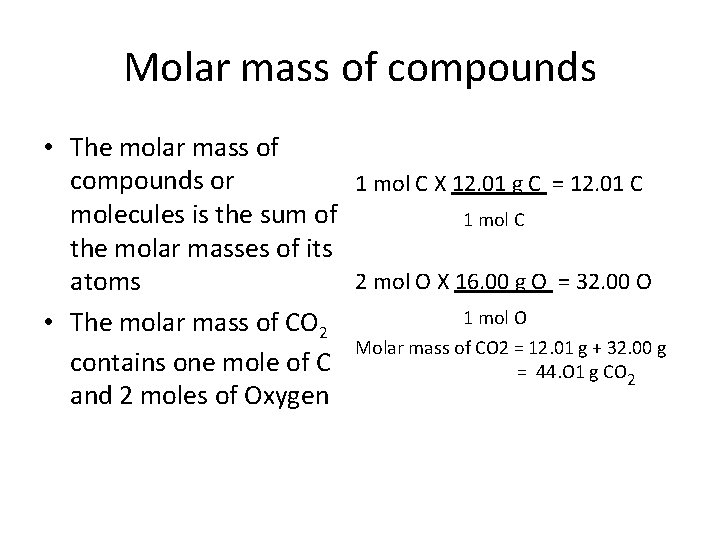
How Many Grams Is 1 Mol Of H20. Homework Statement Suppose you have 6 moles of H20. From 173 mole of water 5184 g of glucose will be produced. Molecular formula of water is H2O Thus one atom of O combines with 2 atoms of H for form 1 molecule of H2O Here atomic weight of O 16 gmol and molecular weight of H2O 18 gmol Now1 mole of O 16 gmol 1 mole of water 18 gmol 25 mole of O 40 gmol 25 moles of water 45 gmol Answer. How many grams are in a mole of h20.
 Molar Mass Butitta Info From studylib.net
Molar Mass Butitta Info From studylib.net
173 moles of water produces 2883 moles of glucose. H2O molecular weight. Since your sample has a mass of 235 g it follows that it will contain. 40 grams H2O to mol 222034 mol. This means it weights 18g per a mole. This means that every 100 g of water will contain a total of 1119 g of hydrogen.
173 moles of water produces 2883 moles of glucose.
Note that rounding errors may occur so always check the results. 1 mole is equal to 1 moles H2O or 1801528. 100 grams H2O to mol 555084 mol. Molar mass of H2O 1801528 gmol. Molecular weight of H20 or grams The SI base unit for amount of substance is the mole. This compound is also known as Water or Dihydrogen Monoxide.
 Source: slideplayer.com
Source: slideplayer.com
173 moles of water produces 2883 moles of glucose. Note that rounding errors may occur so always check the results. 10 grams H2O to mol 055508 mol. However one mole of hydrogen atoms has a mass of 1 gram while one MOLE of hydrogen molecules has a. Meaning 1 mole or 6022 1023 of H2O molecules weighs 18 grams.
 Source: es.pinterest.com
Source: es.pinterest.com
1 grams H2O to mol 005551 mol. 9 is 12 of 18 meaning that 12 mole is present in 9 g of water. 1007942 159994 Percent composition by element. Note that rounding errors may occur so always check the results. This compound is also known as Water or Dihydrogen Monoxide.
 Source: studylib.net
Source: studylib.net
The answer is 0055508435061792. We assume you are converting between moles H2O and gram. 1 grams H20 is equal to 004960612734885 mole. 45 grams of H2O are. 18016gmol x 2mol 36032g.
 Source: slideplayer.com
Source: slideplayer.com
Water has 18 grams per mole so 9 grams is 05 moles of water. 1 mole is equal to 1 moles H2O or 1801528. Considering the perfect formula above MH202 34016gmol. Molecular weight of H20 or grams The SI base unit for amount of substance is the mole. Molar mass of glucose 180 gmol.
 Source: pinterest.com
Source: pinterest.com
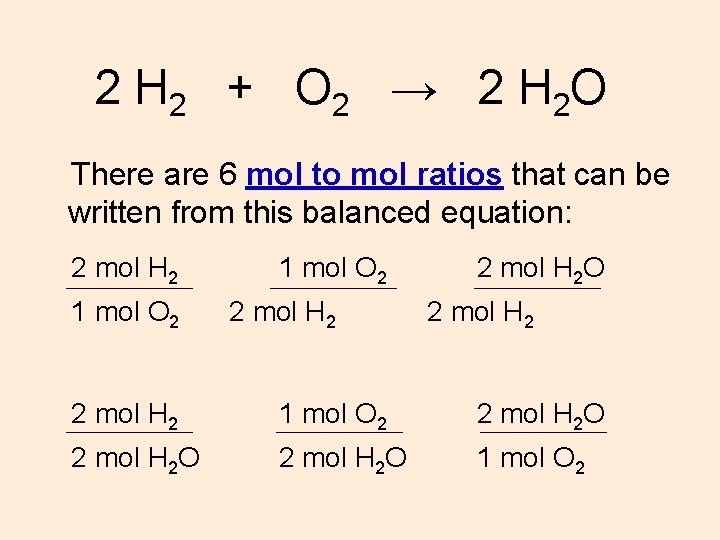
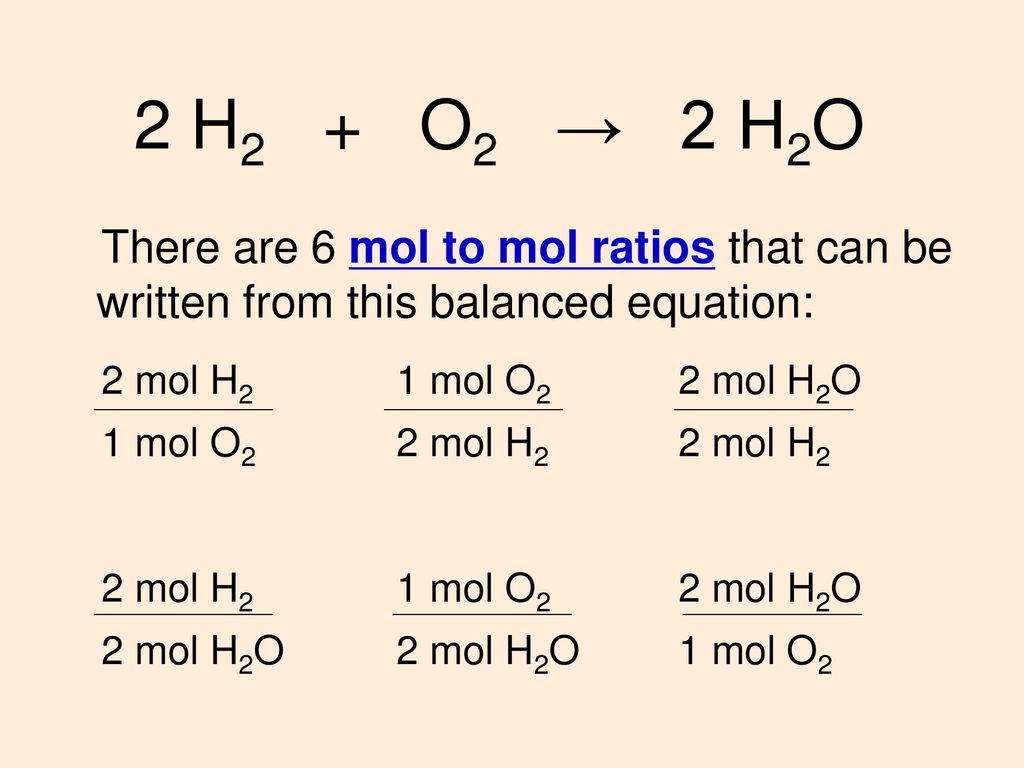
You want to calculate how many moles of H2 you have. 1 grams H2O to mol 005551 mol. 2 100794gmol 180153gmol 100 1119. 1 grams H20 is equal to 004960612734885 mole. 1007942 159994 Percent composition by element.
 Source: slidetodoc.com
Source: slidetodoc.com
You want to calculate how many moles of H2 you have. Note that rounding errors may occur so always check the results. We assume you are converting between grams H20 and mole. Molecular weight of H20 or mol The SI base unit for amount of substance is the mole. 34016gmol x 2mol 68032g.
 Source: slideplayer.com
Source: slideplayer.com
How do you find total atoms. 9 is 12 of 18 meaning that 12 mole is present in 9 g of water. One MOLE of hydrogen atoms contains the same number of atoms as the number of hydrogen molecules in one MOLE of hydrogen molecules ie Avagadros number. 1 mole is equal to 1 moles H20 or 201588 grams. However one mole of hydrogen atoms has a mass of 1 gram while one MOLE of hydrogen molecules has a.
 Source: slideplayer.com
Source: slideplayer.com
1mol of any compound including H20 contains N math602210 math 23 molecules. Meaning 1 mole or 6022 1023 of H2O molecules weighs 18 grams. Molar mass of water 18 gmol. Considering the perfect formula above MH202 34016gmol. 173 moles of water produces 2883 moles of glucose.
 Source: slidetodoc.com
Source: slidetodoc.com
If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. From 173 mole of water 5184 g of glucose will be produced. However one mole of hydrogen atoms has a mass of 1 gram while one MOLE of hydrogen molecules has a. 1 mole is equal to 1 moles H20 or 201588 grams. You want to calculate how many moles of H2 you have.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of H20 or mol The SI base unit for amount of substance is the mole. The technique used can be applied to any mole to gram conversion. You can view more details on each measurement unit. 40 grams H2O to mol 222034 mol. This compound is also known as Water or Dihydrogen Monoxide.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles H20 and gram. The answer is 201588. 10 grams H2O to mol 055508 mol. Note that rounding errors may occur so always check the results. Use this page to learn how to convert between moles H2O and gram.
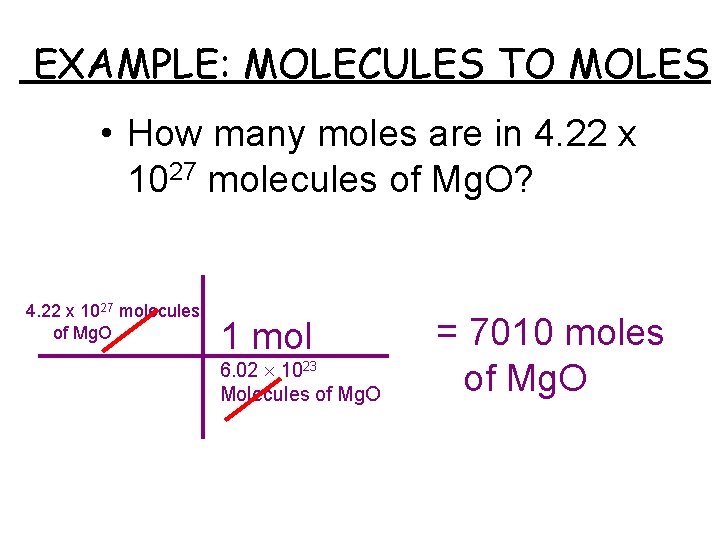 Source: slidetodoc.com
Source: slidetodoc.com
Note that rounding errors may occur so always check the results. In this video well learn to convert moles of H2O to grams. To find the percent composition of hydrogen in water use the molar mass of water and that of hydrogen. 30 grams H2O to mol 166525 mol. The molecular formula for water is H20.
 Source: pinterest.com
Source: pinterest.com
Because you need 6 moles of A and have only 4 moles 3 B chemistry Mg3N2 6H2O- 3MgOH2 2NH3– How many grams of H2O are needed to produce 150g of MgOH2. 10 grams H2O to mol 055508 mol. 1 mole is equal to 1 moles H2O or 1801528 grams. Considering the perfect formula above MH202 34016gmol. Since we know that we have 85 grams of H2O2 it is best we work in grams in order to figure out how many grams of H2O were required to make such an amount.
 Source: slideplayer.com
Source: slideplayer.com
67 g H20 1 1 mol H20 1801528 g H2O 6022e23 molecules H2O 1 mol H2O 224e23 molecules of water. One MOLE of hydrogen atoms contains the same number of atoms as the number of hydrogen molecules in one MOLE of hydrogen molecules ie Avagadros number. 200 grams H2O to mol 1110169 mol. 1 grams H20 is equal to 004960612734885 mole. Homework Equations Avagradros number.
 Source: clutchprep.com
Source: clutchprep.com
12 6022 1023 3011 1023 molecules H2O. Since we know that we have 85 grams of H2O2 it is best we work in grams in order to figure out how many grams of H2O were required to make such an amount. The molecular formula for water is H20. Since we only have 0001 grams that means that we have NA 000118 3351019 molecules of water. 6021023 The Attempt at a Solution I got to the equation.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is 1 mol of h20 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






