Your How many grams in one mole of water images are ready in this website. How many grams in one mole of water are a topic that is being searched for and liked by netizens today. You can Get the How many grams in one mole of water files here. Download all free vectors.
If you’re searching for how many grams in one mole of water images information linked to the how many grams in one mole of water topic, you have visit the ideal site. Our site frequently gives you hints for seeing the maximum quality video and picture content, please kindly search and find more enlightening video articles and graphics that match your interests.
How Many Grams In One Mole Of Water. Divide the mass of water lost when you heated the salt by the molar mass of water roughly 18 grams per mole. 1 mole is equal to 1 moles Water or 1801528 grams. Note that rounding errors may occur so always check the results. The same as the mass of one mole of the compound in grams.
 Converting Grams To Moles Grams To Moles Mass To Moles Mole Conversion From pinterest.com
Converting Grams To Moles Grams To Moles Mass To Moles Mole Conversion From pinterest.com
There is 1 mole of water for every 18 grams of water the atomic mass of oxygen is 16 and the atomic mass of hydrogen is 1 gram so 16 1 1 18. I use N A here as I would any other collective number a dozen or a gross or bakers dozen. You have 36 grams so you have 3618 moles. The molecular formula for Water is H2O. Each H has a mass of 1 and one O has a mass of 16 and that number also represents the number of grams in 1 mole. How many moles of water are in 34g of water.
The SI base unit for amount of substance is the mole.
So in the case of a saturated solution of sucrose in water which is composed of 100 g of sucrose and 473 g of water at 25C the mole fraction of sucrose will be. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. One gram of carbon dioxide contains 136 x 10²¹ number of molecules. How many grams are in a mole. Of molecules in 72 g of water has 4 60231023 molecules. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements.
 Source: slideplayer.com
Source: slideplayer.com
In 1 mole of water there are 6022 1023 moleculesthats how the mole was defined. So in the case of a saturated solution of sucrose in water which is composed of 100 g of sucrose and 473 g of water at 25C the mole fraction of sucrose will be. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. Subsequently question is how many molecules of water are there in 36g of water. How many moles are in 25 grams of water show work.
 Source: pinterest.com
Source: pinterest.com
What is the mass of 1 mole of. Therefore one mole of water weighs 180152 grams. By definition in 1801 g of water 1 mol of water there are Avogadros Number of MOLECULES. Question says 72 g of water. Use this page to learn how to convert between moles Water and gram.
 Source: studylib.net
Source: studylib.net
If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. One gram of carbon dioxide contains 136 x 10²¹ number of molecules. 18 g of water occupies one mole and one mole contains Avagadros no. 1 mole of water weighs 18g. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g.
 Source: youtube.com
Source: youtube.com
Of molecules 60231023 molecules. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. You have 36 grams so you have 3618 moles. In 1 mole of water there are 6022 1023 moleculesthats how the mole was defined. If you take the molecular mass of any molecule in grams then that will be 1 mole of molecules.
 Source: pinterest.com
Source: pinterest.com
How many grams are in a mole. How many molecules are in 1g of co2. The molecular formula for Water is H2O. Fortunately this is another simple calculation. If you take the molecular mass of any molecule in grams then that will be 1 mole of molecules.
 Source: pinterest.com
Source: pinterest.com
So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. Each molecule of water has 3 atoms so the total number of atoms in 10g of water is 33331023. In 1 mole of water there are 6022 1023 moleculesthats how the mole was defined. In our example 16 grams 160 grams per mole 01 moles. To find how many moles of water there are in 18 g we have to divide 18 g by the number of grams in one mole of water also known as the molar mass of water.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles Water or 1801528 grams. 18 g of water occupies one mole and one mole contains Avagadros no. 1 How many moles are in 400 grams of water. How many grams are in a mole. How many moles are in 25 grams of water show work.
 Source: slideplayer.com
Source: slideplayer.com
1 How many moles are in 400 grams of water. The SI base unit for amount of substance is the mole. We assume you are converting between grams Water and mole. So 18 grams is to 1 mol of water as X grams is to 4 moles of water. Its easier to work with grams so convert the mass.
 Source: slideplayer.com
Source: slideplayer.com
One gram of carbon dioxide contains 136 x 10²¹ number of molecules. The answer is 1801528. If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. How many grams are in 1 mole of Fe2O3.
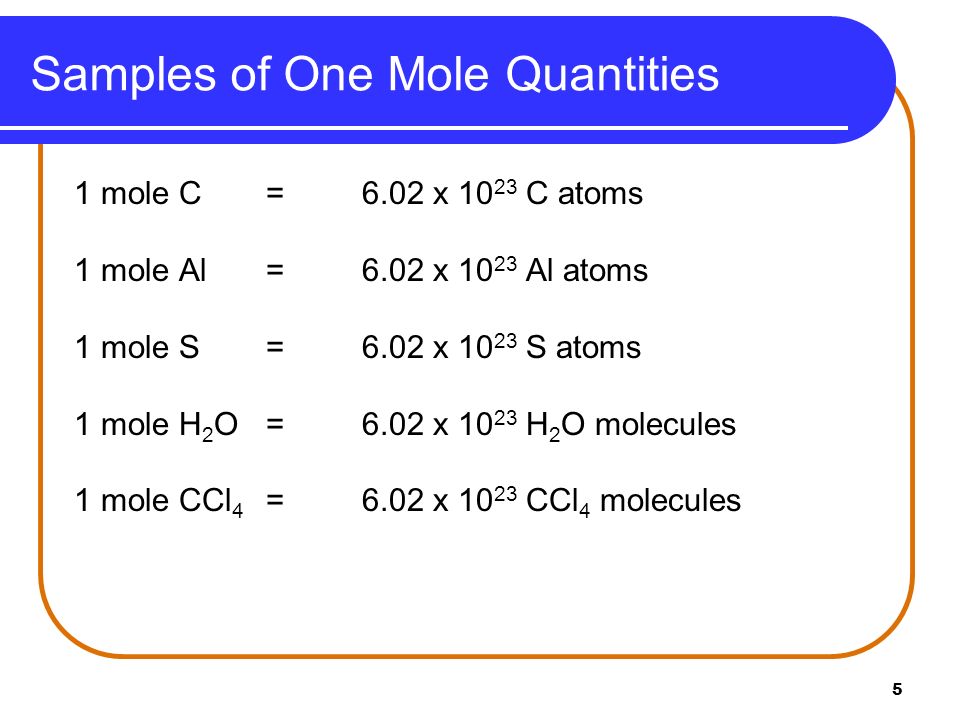 Source: slideplayer.com
Source: slideplayer.com
One gram of carbon dioxide contains 136 x 10²¹ number of molecules. There is 1 mole of water for every 18 grams of water the atomic mass of oxygen is 16 and the atomic mass of hydrogen is 1 gram so 16 1 1 18. Unless you have a good sense of mass this value probably doesnt have much meaning to you. Fortunately this is another simple calculation. Divide the mass of water lost when you heated the salt by the molar mass of water roughly 18 grams per mole.
 Source: pinterest.com
Source: pinterest.com
Of molecules 60231023 molecules. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. Subsequently question is how many molecules of water are there in 36g of water. 1 How many moles are in 400 grams of water. Divide the mass of water lost when you heated the salt by the molar mass of water roughly 18 grams per mole.
 Source: slideplayer.com
Source: slideplayer.com
Unless you have a good sense of mass this value probably doesnt have much meaning to you. Unless you have a good sense of mass this value probably doesnt have much meaning to you. Note that rounding errors may occur so always check the results. The molecular formula for Water is H2O. 72 g of water occupies 4 moles.
 Source: slideplayer.com
Source: slideplayer.com
In 1 mole of water there are 6022 1023 moleculesthats how the mole was defined. So in the case of a saturated solution of sucrose in water which is composed of 100 g of sucrose and 473 g of water at 25C the mole fraction of sucrose will be. The same as the mass of one mole of the compound in grams. How many atoms are in 08288 grams of sodium. How many grams are in 1 mole of Fe2O3.
 Source: slideplayer.com
Source: slideplayer.com
Setting this up as a math problem. How many moles of water are in 34g of water. How many molecules are in 1g of co2. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. The same as the mass of one mole of the compound in grams.
 Source: slideplayer.com
Source: slideplayer.com
Setting this up as a math problem. By definition in 1801 g of water 1 mol of water there are Avogadros Number of MOLECULES. Of molecules 60231023 molecules. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. How many atoms are in 08288 grams of sodium.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams in one mole of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






