Your How many grams in one mole of o2 images are ready. How many grams in one mole of o2 are a topic that is being searched for and liked by netizens now. You can Download the How many grams in one mole of o2 files here. Download all royalty-free images.
If you’re searching for how many grams in one mole of o2 images information linked to the how many grams in one mole of o2 topic, you have pay a visit to the ideal site. Our site always gives you hints for viewing the maximum quality video and image content, please kindly surf and locate more informative video content and graphics that match your interests.
How Many Grams In One Mole Of O2. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. Molecular weight of Oxygen or grams. Click to see full answer. Equivalent of a substance means the weight which combines or displaces 1 g of H2 8 g O2 or 355 g of Cl2.
 Find The Mass Of 2 Moles Of Oxygen Atoms Youtube From youtube.com
Find The Mass Of 2 Moles Of Oxygen Atoms Youtube From youtube.com
Hence the incorrect option is 2. N 5988 g 18015 gmol 3324 mol. Molecular weight of O2 or grams. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 1023 atoms of oxygen. Likewise people ask how many moles are in 16 grams of oxygen. In the given quantity of dioxygen gas there are 2xxN_A oxygen atoms.
Note that rounding errors may occur so always check the results.
Sign In to Writing Essays Science. Use this page to learn how to convert between moles Oxygen and gram. 3 452 grams Argon. What is the mass of 1. Chemistry QA Library How many moles of O2 are in 775 grams. Sign In to Writing Essays Science.
 Source: youtube.com
Source: youtube.com
1 CO2 contains 2 atoms of oxygen hence 1 mole CO2 contains 2 moles of oxygen and therefore moles of oxygen atoms or g-atoms of oxygen are 052 1 g-atom or 1 mole. 1 one mole of O2F2 contains 2 mole of O use. 165 moles 101 grams1 mole16665 grams Hydrogen. Sign In to Tutor. The mass of a mole of Fe2O3 is 2 x Fe 3 x Oxygen 2 x 55845 3 x 1600 15969 grams 1 mole of Fe2O3 15969 grams Fe2O3.
 Source: slideplayer.com
Source: slideplayer.com
From above balanced equation its clear that we need 5 moles of oxygen to burn 1 mole of C3H8. 4 165 moles Hydrogen. Sign In to Tutor. The SI base unit for amount of substance is the mole. The mass of a mole of Fe2O3 is 2 x Fe 3 x Oxygen 2 x 55845 3 x 1600 15969 grams 1 mole of Fe2O3 15969 grams Fe2O3.
 Source: youtube.com
Source: youtube.com
As you already know how the grams to moles conversion work find the number of moles. The mass of a mole of Fe2O3 is 2 x Fe 3 x Oxygen 2 x 55845 3 x 1600 15969 grams 1 mole of Fe2O3 15969 grams Fe2O3. The technique used can be applied to any mole to gram conversion. Knowing how to convert grams to moles may be helpful in numerous chemical tasks eg finding the mole fraction of a solution. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
In the given quantity of dioxygen gas there are 2xxN_A oxygen atoms. 9 g of water. Molecular weight of O2 or grams. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 1023atoms of oxygen. Knowing how to convert grams to moles may be helpful in numerous chemical tasks eg finding the mole fraction of a solution.
 Source: youtube.com
Source: youtube.com
250 moles of Iron. For our practice problem well. 1 CO2 contains 2 atoms of oxygen hence 1 mole CO2 contains 2 moles of oxygen and therefore moles of oxygen atoms or g-atoms of oxygen are 052 1 g-atom or 1 mole. You can always use our grams to moles calculator to check the result. N 5988 g 18015 gmol 3324 mol.
 Source: slideplayer.com
Source: slideplayer.com
224 g of hydrogen reacts with 13232 g of oxygen to form 21836 g of water. In this video well learn to convert moles of O2 to grams. Write the expression for the moles of the oxygen. In other words 1 mole of oxygen would contain molecules. Molecular weight of O2 or grams.
 Source: youtube.com
Source: youtube.com
Beside this how many gram atoms are present in 256g of o2. 452 grams 1 mole39948 grams11315 moles Argon. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. To put this another way a wholesaler buys 100 pairs of gloves. One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms.
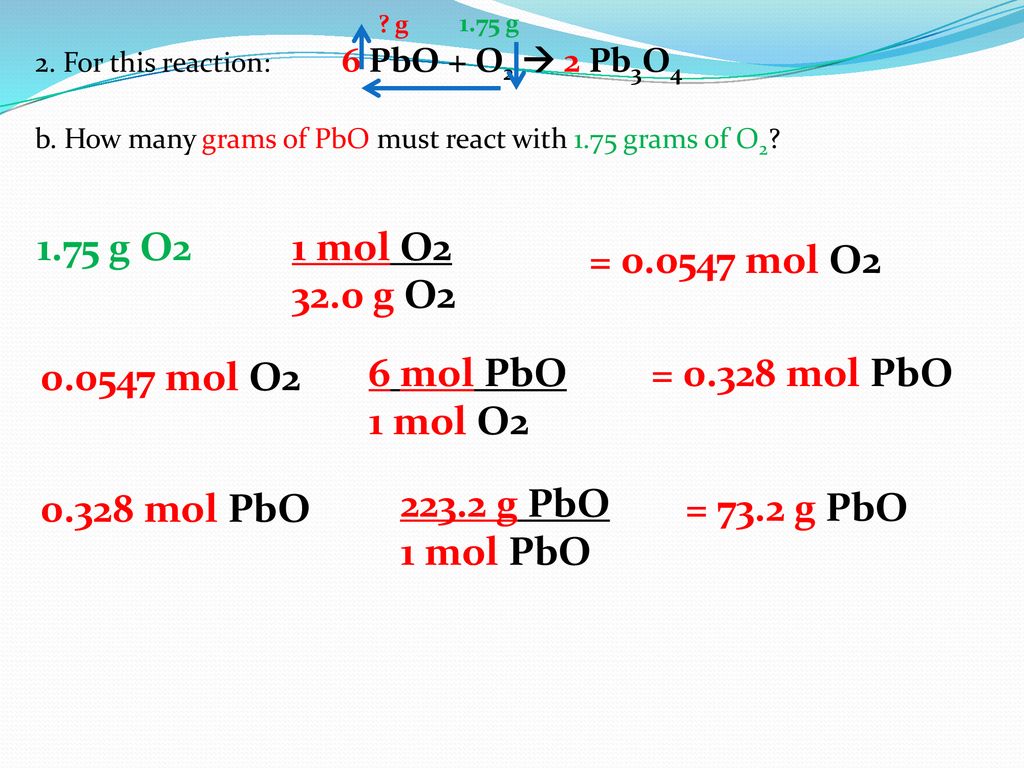 Source: slideplayer.com
Source: slideplayer.com
As you already know how the grams to moles conversion work find the number of moles. For those elements that do not occur as single atoms - that is the diatomic gases sulfur and phosphorus - it is important to be certain that you specify what you are talking about. 1 mole is equal to 1 moles O2 or 319988 grams. 28 grams 1 mole16 grams per mole175 moles oxygen. Knowing how to convert grams to moles may be helpful in numerous chemical tasks eg finding the mole fraction of a solution.
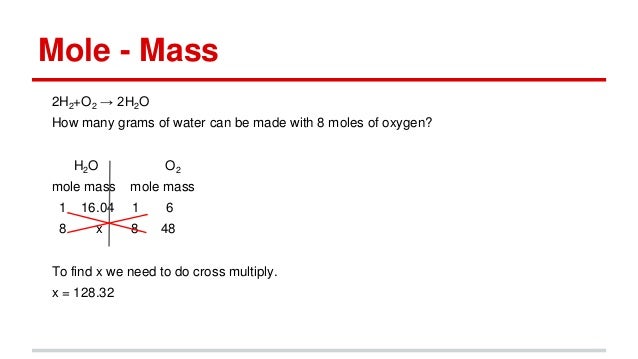 Source: slideshare.net
Source: slideshare.net
Use this page to learn how to convert between moles Oxygen and gram. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Besides how many moles are in 16 grams of oxygen. 250 moles of Iron. 9 g of water.
 Source: slideplayer.com
Source: slideplayer.com
Equivalent of a substance means the weight which combines or displaces 1 g of H2 8 g O2 or 355 g of Cl2. Equivalent of a substance means the weight which combines or displaces 1 g of H2 8 g O2 or 355 g of Cl2. In 1 mol O_2 gas by definition you have Avogadros number of MOLECULES. Mass of 50 moles of oxygen 50moles 32 g mol 1600 g 16 kg. For our practice problem well.
 Source: youtube.com
Source: youtube.com
In other words 1 mole of oxygen would contain molecules. 1 mole is equal to 1 moles O2 or 319988 grams. 9 g of water. The number of molecules in 16 gm of oxygen are 05 1632 moles. Avogadros number N_A 6022140857xx1023.
 Source: slideplayer.com
Source: slideplayer.com
As you already know how the grams to moles conversion work find the number of moles. Note that rounding errors may occur so always check the results. The SI base unit for amount of substance is the mole. 1 10 -6. Sign In to Tutor.
 Source: toppr.com
Source: toppr.com
9 g of water. In other words 1 mole of oxygen would contain molecules. Use this page to learn how to convert between moles O2 and gram. How many GRAMS of oxygen are present in 167 moles of dioxygen difluoride O2F2. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules.
 Source: youtube.com
Source: youtube.com
250 moles of Iron. Click to see full answer. 3 452 grams Argon. 1 10 -6. N 5988 g 18015 gmol 3324 mol.
 Source: youtube.com
Source: youtube.com
One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 10 23 atoms of oxygen. Therefore 256 g of oxygen O2 has 963x 1024 atoms of oxygens. Click to see full answer. 320 grams 1 mol 320 grams 1 mol. Thus 4 moles of oxygen gas O2 would have a mass of 128 g.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams in one mole of o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






