Your How many grams in one mole of n2 images are ready. How many grams in one mole of n2 are a topic that is being searched for and liked by netizens today. You can Get the How many grams in one mole of n2 files here. Get all free photos and vectors.
If you’re searching for how many grams in one mole of n2 pictures information connected with to the how many grams in one mole of n2 interest, you have visit the ideal site. Our site always gives you suggestions for refferencing the maximum quality video and image content, please kindly hunt and locate more enlightening video content and graphics that fit your interests.
How Many Grams In One Mole Of N2. Indeed one can work out what the base units of length and mass have to be in order for all the practical units to be coherent the watt and the joule as well as the volt the ampere etc. How many grams of Nal are produced per litre of N2g formed in the decomposition of sodium azide NaN3 if the gas is collected at 25 degrees celsius and 10 bar 98692 atm. 7800 is to 0167 as the total pressure is to one so 468 mmHg is the answer. How many grams of argon must be pumped into the flask in order to make the partial pressure of argon twice that of helium.
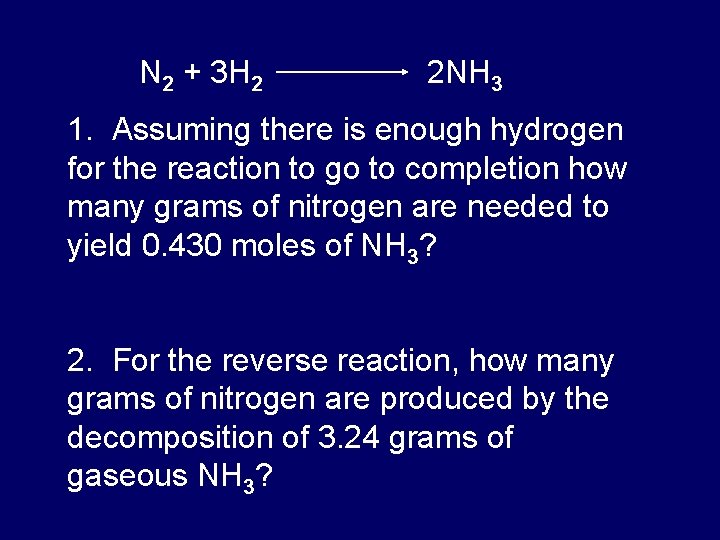 Quick Review The Mole A Very Large Counting From slidetodoc.com
Quick Review The Mole A Very Large Counting From slidetodoc.com
20 120 0167 of the total pressure. How many grams of argon must be pumped into the flask in order to make the partial pressure of argon twice that of helium. It is equal to one mole of atoms. How many grams of nitrogen gas are present in this sample. Assume those conditions for this question. How many grams of mercury are present in 225 g of pure HgS.
How Many Grams are There in One Mole.
2C2H6g7O2g4CO2g6H2OlHow many liters of carbon dioxide will be produced when 179 L of ethane are burned. It is equal to one mole of atoms. How many grams of Li3N must you react with excess water. Instead of you having to figure out the grams of SO 2 in one mole I will tell you that it is 641g SO 2 1 mole SO 2. Molar mass of Hg and S are 2006 g mol-1 and 32 g mol-1 respectively. Indeed one can work out what the base units of length and mass have to be in order for all the practical units to be coherent the watt and the joule as well as the volt the ampere etc.
 Source: pinterest.com
Source: pinterest.com
How is it related to mole and Avogadro number. It is equal to one mole of atoms. N2 is collected in a100 mL container at a pressure of 688 mm Hg and a temperature of 565 C. Calculate mass of Nitrogen N2 which contains same number of molecules as are present in 44 grams of Carbon-di-oxide CO2. Iron given atomic mass of.
 Source: youtube.com
Source: youtube.com
2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O. 20 120 0167 of the total pressure. Nitrogen exists as N2 diatomic. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O. 18531932 introduced the word mole in 1896 as follows one mole as molecular weight of a substance in grams In 1969 International Committee on Weights and Measures defined the mole as the amount of a substance that contains as many elementary.
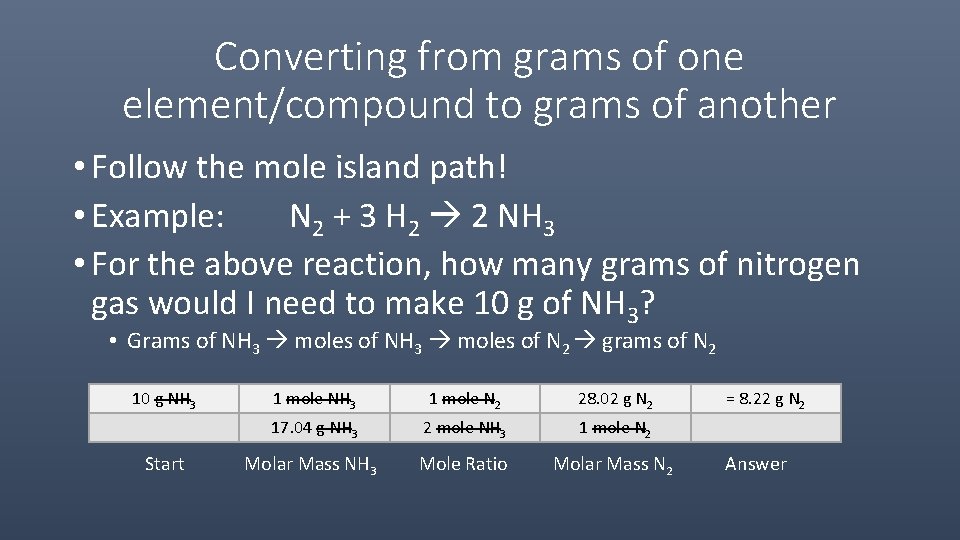 Source: slidetodoc.com
Source: slidetodoc.com
One mole of any gas occupies 224 L under certain conditions of temperature and pressure. Iron given atomic mass of. How many grams of argon must be pumped into the flask in order to make the partial pressure of argon twice that of helium. For example in the reaction. One gram atomic mass 6022 1023 atoms 1 mole.
 Source: youtube.com
Source: youtube.com
One gram atomic mass 6022 1023 atoms 1 mole. As explained in Introduction to Chemical Engineering. 18531932 introduced the word mole in 1896 as follows one mole as molecular weight of a substance in grams In 1969 International Committee on Weights and Measures defined the mole as the amount of a substance that contains as many elementary. Mole Wilhelm Ostwald LatvianGerman chemist and a 1909 Nobel prize laureate. The lowest-energy configuration of the dioxygen molecule is a stable relatively unreactive diradical in a triplet spin stateBonding can be described with three bonding electron.
 Source: slidetodoc.com
Source: slidetodoc.com
The R constant is 008206 LatmKmol. Combustion in oxygen is a chain reaction in which many distinct radical intermediates participate. You can determine the molecular weight by looking up the numbers under the element names in the periodic table. N2 2H2 arrow N2H4. The high energy required for initiation is explained by the unusual structure of the dioxygen molecule.
 Source: youtube.com
Source: youtube.com
Set up the equation. One mole of any gas occupies 224 L under certain conditions of temperature and pressure. One gram atomic mass 6022 1023 atoms 1 mole. Set up the equation. Mole Wilhelm Ostwald LatvianGerman chemist and a 1909 Nobel prize laureate.
 Source: slideplayer.com
Source: slideplayer.com
How many grams of mercury are present in 225 g of pure HgS. N2 2H2 arrow N2H4. How many grams of N2 are required to make 7832 grams of hydrazine. The values are 10 7 metres one half of a meridian of the Earth called a quadrant and 10 11 grams called an eleventh-gram. Mole Wilhelm Ostwald LatvianGerman chemist and a 1909 Nobel prize laureate.
 Source: toppr.com
Source: toppr.com
How many grams of N2 are required to make 7832 grams of hydrazine. One gram atomic mass 6022 1023 atoms 1 mole. Instead of you having to figure out the grams of SO 2 in one mole I will tell you that it is 641g SO 2 1 mole SO 2. As explained in Introduction to Chemical Engineering. For example in the reaction.
 Source: gr.pinterest.com
Source: gr.pinterest.com
How many grams of N2 are required to make 7832 grams of hydrazine. Combustion in oxygen is a chain reaction in which many distinct radical intermediates participate. The lowest-energy configuration of the dioxygen molecule is a stable relatively unreactive diradical in a triplet spin stateBonding can be described with three bonding electron. One gram atomic mass 6022 1023 atoms 1 mole. One mole of H2S gas escapes from a container by effusion in 77 seconds.
 Source: gr.pinterest.com
Source: gr.pinterest.com
Assume those conditions for this question. The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O. Na 23 u Fe 56 u. A flask contains 200 moles of nitrogen and 200 moles of helium. One mole of hydroxybutyric.

The atomic mass of an element expressed in grams is called gram atomic mass. Define the term gram atom. Molar mass of Hg and S are 2006 g mol-1 and 32 g mol-1 respectively. How many grams of N2 are required to make 7832 grams of hydrazine. A sample of nitrogen gas N2 contains 12 1024 atoms of nitrogen.
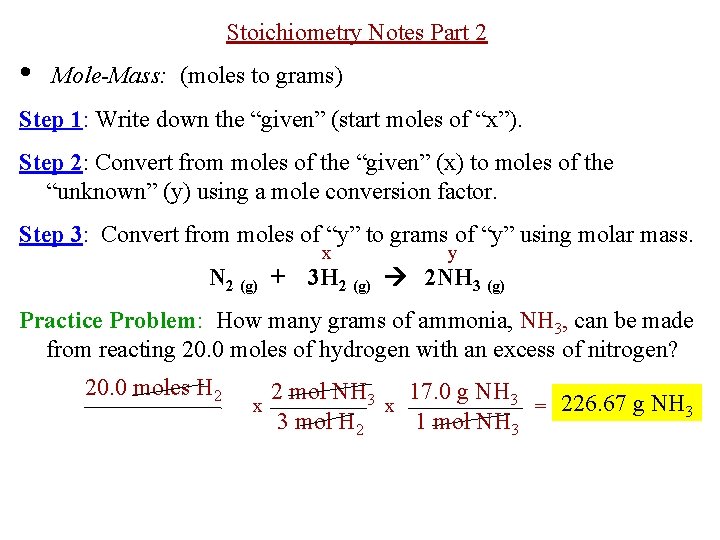 Source: slidetodoc.com
Source: slidetodoc.com
One gram atom of any element contains 6022 1023 atoms of the element. How many grams of nitrogen gas are present in this sample. How is it related to mole and Avogadro number. It is equal to one mole of atoms. How many grams of Li3N must you react with excess water.
 Source: youtube.com
Source: youtube.com
One mole of any gas occupies 224 L under certain conditions of temperature and pressure. Tools for Today and Tomorrow 5th Edition we select that number because it is convenient and because it makes it easy to calculate how many sigmol of each substance are present in that quantity of material. It is equal to one mole of atoms. N2 is collected in a100 mL container at a pressure of 688 mm Hg and a temperature of 565 C. As explained in Introduction to Chemical Engineering.
 Source: youtube.com
Source: youtube.com
Calculate mass of Nitrogen N2 which contains same number of molecules as are present in 44 grams of Carbon-di-oxide CO2. Assume those conditions for this question. Which has more number of atoms 100 grams of sodium or 100 grams of. A flask contains 200 moles of nitrogen and 200 moles of helium. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O.
 Source: youtube.com
Source: youtube.com
A flask contains 200 moles of nitrogen and 200 moles of helium. 2C2H6g7O2g4CO2g6H2OlHow many liters of carbon dioxide will be produced when 179 L of ethane are burned. How many moles of gold are present in an ornament containing 8865 grams of gold. 18531932 introduced the word mole in 1896 as follows one mole as molecular weight of a substance in grams In 1969 International Committee on Weights and Measures defined the mole as the amount of a substance that contains as many elementary. You can determine the molecular weight by looking up the numbers under the element names in the periodic table.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams in one mole of n2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






