Your How many grams in one mole of h20 images are ready in this website. How many grams in one mole of h20 are a topic that is being searched for and liked by netizens today. You can Download the How many grams in one mole of h20 files here. Get all royalty-free photos and vectors.
If you’re looking for how many grams in one mole of h20 pictures information related to the how many grams in one mole of h20 topic, you have come to the right site. Our site always gives you suggestions for seeking the highest quality video and image content, please kindly hunt and locate more enlightening video articles and graphics that match your interests.
How Many Grams In One Mole Of H20. How many is it. 1 mole of oxygen atoms got 16 grams. Homework Statement Suppose you have 6 moles of H20. We know in one mole of water H₂O one mole of oxygen atoms present.
 Chapter 8 The Mole Part 1 Ppt Download From slideplayer.com
Chapter 8 The Mole Part 1 Ppt Download From slideplayer.com
Note that rounding errors may occur so always check the results. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. 2 moles of H2O gets you 36 grams. The SI base unit for amount of substance is the mole. Convert the mass to moles using molar mass. 20 grams H2O to mol 111017 mol.
The SI base unit for amount of substance is the mole.
135 x 103 molese. 9 is 12 of 18 meaning that 12 mole is present in 9 g of water. One MOLE of hydrogen atoms contains the same number of atoms as the number of hydrogen molecules in one MOLE of hydrogen molecules ie Avagadros number. 50 grams H2O to mol 277542 mol. One gram of H20 is close to 006 moles. 50 grams 10 H2O to mol 178474 mol.
 Source: beyondmediocre.net
Source: beyondmediocre.net
This compound is also known as Water or Dihydrogen Monoxide. What is the mass of water in grams. So number of moles of water 2 moles. 2 moles of H2O gets you 36 grams. 1 mole is equal to 1 moles H20 or 201588 grams.
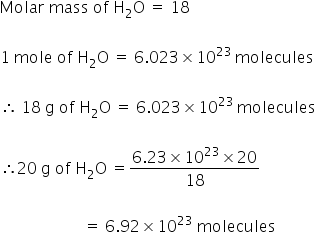 Source: topperlearning.com
Source: topperlearning.com
From the Periodic Table of Elements one sees that one mole of hydrogen atoms weighs 1 gram while one mole of oxygen atoms weighs 16 grams. Homework Statement Suppose you have 6 moles of H20. So 1 g water will contain 118 moles of H2O molecules. Were being asked to calculate how many moles of water are there in 100 grams of water. Note that rounding errors may occur so always check the results.

This compound is also known as Water or Dihydrogen Monoxide. 30 grams 10 H2O to mol 107084 mol. 1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams. 1 mole of oxygen atoms got 16 grams. Molecular weight of H20 or grams.
 Source: slideplayer.com
Source: slideplayer.com
20 grams 10 H2O to mol 07139 mol. 6021023 The Attempt at a Solution I got to the equation. 100 grams 10 H2O to mol 356948 mol. One gram of H20 is close to 006 moles. 1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams.

Hope you got it. Mass 30g so. Convert grams H2O to moles or moles H2O to grams. The molar mass of H20 is about 18 gmol. 40 grams 10 H2O to mol 142779 mol.
 Source: clutchprep.com
Source: clutchprep.com
The answer is 1665253. And since the chemical formula for water is H 2O 1 mole of water corresponds to 1. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. 200mol 602 1023molecules 1mol 1204 1024molecules. So number of moles of water 2 moles.
 Source: slideplayer.com
Source: slideplayer.com
Type in your own numbers in the form to convert the units. How many moles are in 25 grams of water show work. In 1 mole of water there are 6022 1023 moleculesthats how the mole was defined. 200 grams 10 H2O to mol 713896 mol. Thus 36g of water is equivalent to 12 1024molecules.
 Source: slideserve.com
Source: slideserve.com
One gram of H20 is close to 006 moles. How many moles are 100 grams of water. They do not affect the number of significant figures. For our practice problem well. You want to calculate how many moles of H2 you have.
 Source: slideplayer.com
Source: slideplayer.com
9 is 12 of 18 meaning that 12 mole is present in 9 g of water. Thus 36g of water is equivalent to 12 1024molecules. Convert the mass to moles using molar mass. They do not affect the number of significant figures. Number of moles 18 g 1008 gmol 2 1600 gmol 18 g 1802 gmol 1 mole.
 Source: slideplayer.com
Source: slideplayer.com
Since your sample has a mass of 235 g it follows that it will contain. This means 17 g of ammonia gas is equivalent to 1 mole of ammonia gas molecule. However one mole of hydrogen atoms has a mass of 1 gram while one MOLE of. Note that rounding errors may occur so always check the results. 200 grams H2O to mol 1110169 mol.
 Source: youtube.com
Source: youtube.com
So is equal to 18 moles. However one mole of hydrogen atoms has a mass of 1 gram while one MOLE of. 10 grams 10 H2O to mol 035695 mol. How many is it. In this video well learn to convert moles of H2O to grams.
 Source: youtube.com
Source: youtube.com
2 100794gmol 180153gmol 100 1119. Does 100mL 100g weigh. 100 grams H2O to mol 555084 mol. Convert grams H2O to moles or moles H2O to grams. One gram of H20 is close to 006 moles.
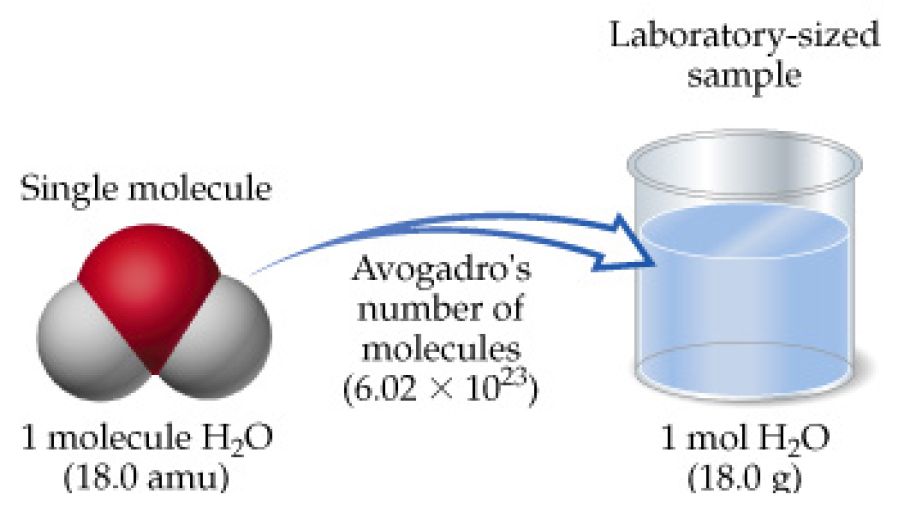 Source: socratic.org
Source: socratic.org
Use this page to learn how to convert between moles H20 and gram. To find the percent composition of hydrogen in water use the molar mass of water and that of hydrogen. 200 grams 10 H2O to mol 713896 mol. Number of moles 18 g 1008 gmol 2 1600 gmol 18 g 1802 gmol 1 mole. The technique used can be applied to any mole to gram conversion.
 Source: slideplayer.com
Source: slideplayer.com
Answer 1 of 4. 1 mole is equal to 1 moles H20 or 201588 grams. 1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams. If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. The answer is 1665253.
 Source: slideplayer.com
Source: slideplayer.com
The molar mass of water is 1802 gmol. We assume you are converting between moles H2O and gram. 1 mole is equal to 1 moles H2O or 1801528 grams. 40 grams 10 H2O to mol 142779 mol. 12 6022 1023 3011 1023 molecules H2O.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams in one mole of h20 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






