Your How many grams in 1 mole of o2 images are ready in this website. How many grams in 1 mole of o2 are a topic that is being searched for and liked by netizens now. You can Get the How many grams in 1 mole of o2 files here. Download all royalty-free photos.
If you’re searching for how many grams in 1 mole of o2 pictures information linked to the how many grams in 1 mole of o2 topic, you have visit the right site. Our website frequently gives you suggestions for refferencing the highest quality video and image content, please kindly surf and locate more informative video articles and graphics that fit your interests.
How Many Grams In 1 Mole Of O2. Correspondingly how many moles are in 16 grams of oxygen. The answer is 0062502343837894. In other words 1 mole of oxygen would contain molecules. Find the number of moles of argon in 452 g of argon.
 Information Given By Chemical Equations Ppt Video Online Download From slideplayer.com
Information Given By Chemical Equations Ppt Video Online Download From slideplayer.com
The answer would be 1875A Explanation. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 1023 oxygen atoms. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie.
Now we can do the same for the other two compounds.
For every mole of H2SO4 molecules there are 4 moles of oxygen atoms which has a molar mass of 1600 gmol mass of the part. The molecular mass of water H2O is 18 12 16 so one mole of water would be 18 grams. Besides how many moles are in 16 grams of oxygen. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. View the full answer. Each O2 has two oxygen atoms hence the 2 and there are Avogadros number in a mole so 2 X Avogadros number is the answer - a lot.
 Source: youtube.com
Source: youtube.com
Number of moles of O 2 number of moles of O2F2 2 167 334 mol Mol. We know this by the very definition of mole concept. Molecular weight of Oxygen or grams The molecular formula for Oxygen is O. Here the molar mass of oxygen is M O2 M O 2 and the mass is m. For our practice problem well.
 Source: youtube.com
Source: youtube.com
Molecular weight of Oxygen or grams The molecular formula for Oxygen is O. In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³. We know this by the very definition of mole concept. Therefore 1 gram molecule of oxygen represents one mole of O2 gas. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
Answer 1 of 5. Therefore 1 gram molecule of oxygen represents one mole of O2 gas. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 10 23 atoms of oxygen. If you take the molecular mass of any molecule in grams then that will be 1 mole of molecules. We assume you are converting between moles O2 and gram.
 Source: slideplayer.com
Source: slideplayer.com
Now we just punch in the numbers and find. Answer 1 of 2. The number of molecules in 16 gm of oxygen are 05 1632 moles. If you are still confused I would recommend Khan AcademyThey are great at teaching chemistry. Find the grams in 165 mol of Hydrogen.
 Source: slideshare.net
Source: slideshare.net
Of moles of oxygen atoms in 1 mole of calcium nitrate 6 So no. How many atoms are in 1g of oxygen. In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³. The molecular mass of water H2O is 18 12 16 so one mole of water would be 18 grams. In the given quantity of dioxygen gas there are 2xxN_A oxygen atoms.
 Source: slideplayer.com
Source: slideplayer.com
The number of molecules in 16 gm of oxygen are 05 1632 moles. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 1023 oxygen atoms. Each O2 has two oxygen atoms hence the 2 and there are Avogadros number in a mole so 2 X Avogadros number is the answer - a lot. Number of moles of O 2 number of moles of O2F2 2 167 334 mol Mol. Here the molar mass of oxygen is M O2 M O 2 and the mass is m.
 Source: slideplayer.com
Source: slideplayer.com
Likewise people ask how many moles are in 16 grams of oxygen. How many grams are in one mole of Ca NO3 2. Of moles of oxygen atoms in 25 moles 625 15 Mass of 1 mole of oxygen atoms. Since oxygen has an atomic mass of 16 gmole the molar mass of oxygen gas O2 is 2 x 16 gmole 32 gmole. What is the 1 mole of oxygen.
 Source: youtube.com
Source: youtube.com
Here we are given with 16 g of oxygen. View the full answer. You can view more details on each measurement unit. How many GRAMS of oxygen are present in 167 moles of dioxygen difluoride O2F2. The number of molecules in 16 gm of oxygen are 05 1632 moles.
 Source: slideserve.com
Source: slideserve.com
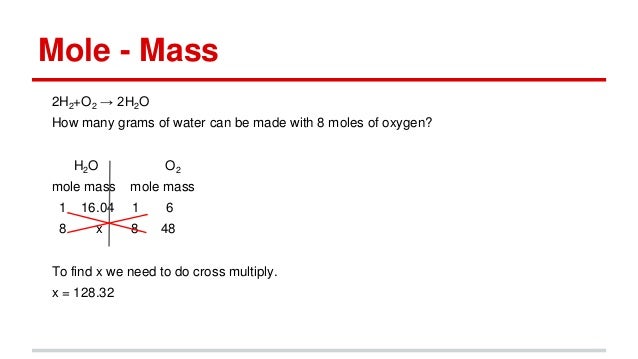
Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. For every mole of H2SO4 molecules there are 4 moles of oxygen atoms which has a molar mass of 1600 gmol mass of the part. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. What is the 1 mole of oxygen. The answer would be 1875A Explanation.

The number of molecules in 16 gm of oxygen are 05 1632 moles. View the full answer. Avogadros number N_A 6022140857xx1023. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. The technique used can be applied to any mole to gram conversion.
 Source: youtube.com
Source: youtube.com
We assume you are converting between moles O2 and gram. Density of oxygen is equal to 1429 kgm³. How many moles of oxygen is. Of moles of oxygen atoms in 25 moles 625 15 Mass of 1 mole of oxygen atoms. Molecular weight of Oxygen or grams The molecular formula for Oxygen is O.
 Source: youtube.com
Source: youtube.com
Number of moles of O 2 number of moles of O2F2 2 167 334 mol Mol. Thus 4 moles of oxygen gas O2 would have a mass of 128 g. Of moles of oxygen atoms in 25 moles 625 15 Mass of 1 mole of oxygen atoms. How many GRAMS of oxygen are present in 167 moles of dioxygen difluoride O2F2. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 1023 oxygen atoms.
 Source: slideplayer.com
Source: slideplayer.com
One gram molecule represents one mole molecules of oxygen gas. Here we are given with 16 g of oxygen. How many individual gloves does he buy. The answer would be 1875A Explanation. Thus 4 moles of oxygen gas O2 would have a mass of 128 g.
 Source: toppr.com
Source: toppr.com
Each O2 has two oxygen atoms hence the 2 and there are Avogadros number in a mole so 2 X Avogadros number is the answer - a lot. For our practice problem well. How many grams are in one mole of Ca NO3 2. Now we can do the same for the other two compounds. Find the number of moles of argon in 452 g of argon.
 Source: youtube.com
Source: youtube.com
Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Of moles of oxygen atoms in 1 mole of calcium nitrate 6 So no. What is 1g of oxygen. Find the grams in 165 mol of Hydrogen. Here we are given with 16 g of oxygen.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams in 1 mole of o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






