Your How many grams in 1 mole of nacl images are available in this site. How many grams in 1 mole of nacl are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams in 1 mole of nacl files here. Download all royalty-free photos.
If you’re looking for how many grams in 1 mole of nacl images information connected with to the how many grams in 1 mole of nacl keyword, you have come to the right blog. Our site frequently gives you suggestions for refferencing the maximum quality video and picture content, please kindly hunt and find more informative video articles and graphics that match your interests.
How Many Grams In 1 Mole Of Nacl. Note that rounding errors may occur so always check the results. How many grams are in 1 mole of nacl. The SI base unit for amount of substance is the mole. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements.
 1 Determine The Gram Formula Mass Of A Compound 2 Convert Between Grams And Moles 3 Convert Between Moles And Grams Ppt Download From slideplayer.com
1 Determine The Gram Formula Mass Of A Compound 2 Convert Between Grams And Moles 3 Convert Between Moles And Grams Ppt Download From slideplayer.com
So multiply 1946 by 5844 and you get 11372424grams dont know how far. Number of moles To find the molar mass of NaCl. For our practice problem wel. Molecular weight of NaCl or grams. One mol of NaCl 602 x1023 formulas has a mass of 5844 g. To find the grams when you have moles you have to multiply the moles by molar mass of NaCl.
600 g58443 gmol 1027 mol of NaCl.
Molecular weight of NaCl or grams. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. Then multiply by the quantity 14 liter 250 cm3 to get 18 moles NaCl. 124 1024 formula units. So multiply 1946 by 5844 and you get 11372424grams dont know how far.
 Source: youtube.com
Source: youtube.com
So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. Molecular weight of NaCl or mol. Then multiply by the quantity 14 liter 250 cm3 to get 18 moles NaCl. Molar mass of Cl 35453 gmol Cl. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
Then multiply by the quantity 14 liter 250 cm3 to get 18 moles NaCl. In this video well learn to convert moles of NaCl to grams. How many grams are in 150 moles of KMnO4. This compound is also known as Sodium Chloride. One mol of NaCl 602 x1023 formulas has a mass of 5844 g.
 Source: slideplayer.com
Source: slideplayer.com
Click hereto get an answer to your question How many moles and how many grams of NaCl are present in 250 mL of a 05 M NaCl solution. It is made up of sodium ions Na and chloride ions Cl. Herein how many mEq are in a mg of sodium chloride. Total molar mass 58442 gmol NaCl. As stated 1 mole of NaCl weighs 5844 g.
 Source: pinterest.com
Source: pinterest.com
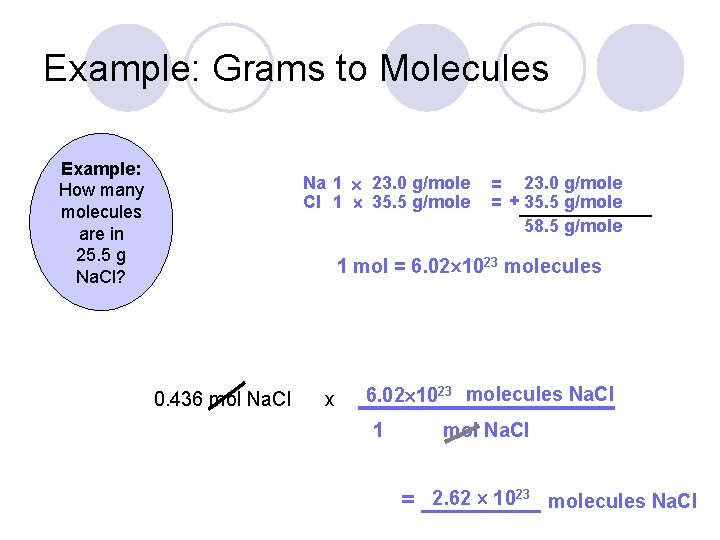
To find the grams when you have moles you have to multiply the moles by molar mass of NaCl. 1 mol of NaCl 602 1023 formula units. 100 g NaCl 1 mol NaCl5844 g NaCl 1 mol Na 1 mol NaCl 2299 g of Na 1 mol Na which equals 39339435 g of Na. To obtain one mole of copper atoms 602 x 1023 atoms weigh out 6355 g copper. In this video well learn to convert moles of NaCl to grams.
 Source: pinterest.com
Source: pinterest.com
600 g58443 gmol 1027 mol of NaCl. Molar mass of Cl 35453 gmol Cl. What number of moles of NaCl are wanted to arrange 150 liters of 0250 M answer. The relation between molecular formula mass and molar mass. Molecular weight of NaCl or grams.
 Source: youtube.com
Source: youtube.com
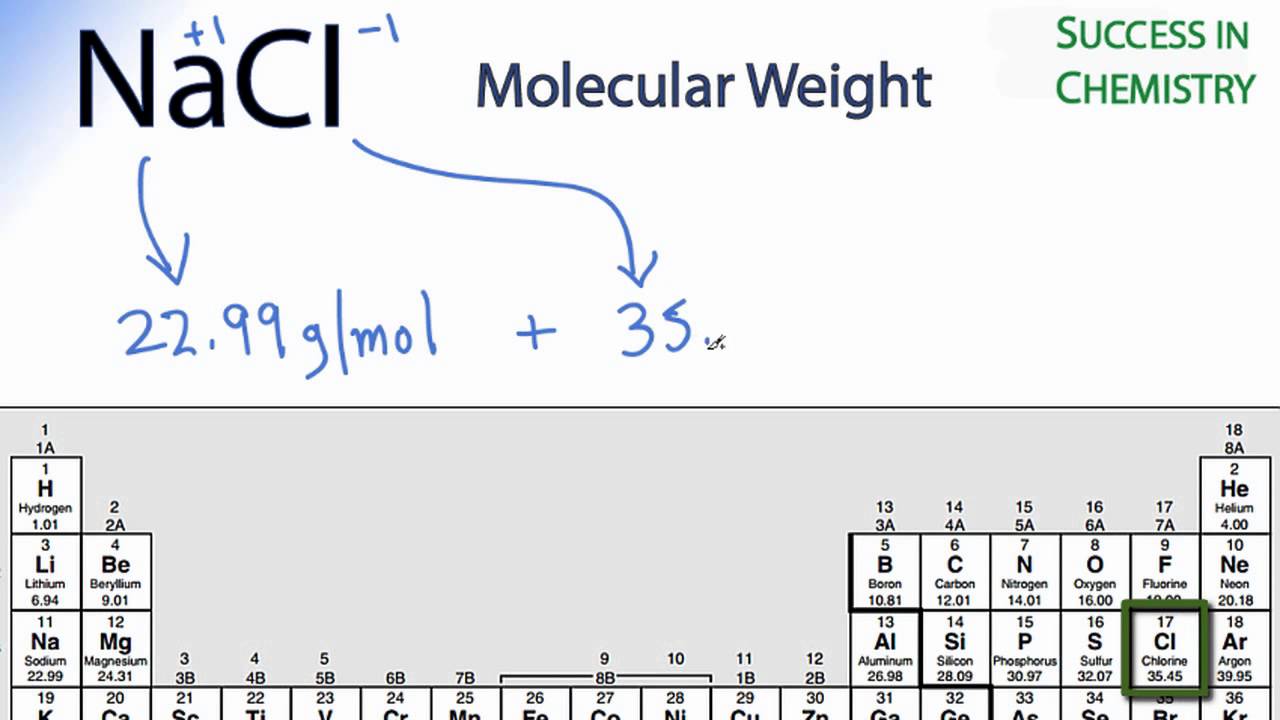
For our practice problem wel. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. 124 1024 formula units. 1 mole of NaCl is 5844 g 200 g NaCl 1 mol NaCl5844 g NaCl 3422 mol NaCl There are about 34 moles in 200 grams of NaCl. The atomic mass of Na 23g atomic mass of Cl 355g Molar mass of NaCl 23 355 585gmol¹.
 Source: youtube.com
Source: youtube.com
A 002 M solution contains 002 moles per liter. One mol of NaCl 602 x1023 formulas has a mass of 5844 g. 1 mol of NaCl 602 1023 formula units. You can view more details on each measurement unit. Note that rounding errors may occur so always check the results.
 Source: youtube.com
Source: youtube.com
One molecule of water H2O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a mole of water molecules would weigh 1802 grams. 5844 gmol is the molecular weight of NaCl. Molecular weight of NaCl or grams. 1 mole is equal to 1 moles KMnO4 or 158033949 grams. To obtain one mole of copper atoms 602 x 1023 atoms weigh out 6355 g copper.
 Source: studylib.net
Source: studylib.net
Formula units NaCl 120 g NaCl 1 mol NaCl 5844 g NaCl 602 1023 formula units 1 mol NaCl. The relation between molecular formula mass and molar mass. 1 mol of NaCl 602 1023 formula units. What number of moles of NaCl are wanted to arrange 150 liters of 0250 M answer. In this video well learn to convert moles of NaCl to grams.
 Source: pinterest.com
Source: pinterest.com
600 g58443 gmol 1027 mol of NaCl. If you need to maintain. What number of moles of NaCl are wanted to arrange 150 liters of 0250 M answer. NaCls molar mass is Na- 2299 Cl-3545 atomic masses 5844. When serum sodium ranges between 150 and 160 mEql 1 mEq milliequivalent is equal one mM of sodium 23 mg.
 Source: youtube.com
Source: youtube.com
One gram of NaCl contains 172 mEq of sodium which corresponds to 9-10 gl of NaCl the central nervous system CNS symptoms are common and seizures occur in approximately. How many moles and how many grams of NaCl are present in 250 mL of a 05 M NaCl solution. The SI base unit for quantity of substance is the mole. Use this page to learn how to convert between moles NaCl and gram. Sodium chloride is NaCl.
 Source: slideplayer.com
Source: slideplayer.com
Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. How many grams are in 150 moles of KMnO4. As stated 1 mole of NaCl weighs 5844 g. 1 mol of NaCl 602 1023 formula units. In this video well learn to convert moles of NaCl to grams.
 Source: slidetodoc.com
Source: slidetodoc.com
Number of moles To find the molar mass of NaCl. To go from grams to. Molecular weight of NaCl or mol. Herein how many mEq are in a mg of sodium chloride. It is made up of sodium ions Na and chloride ions Cl.
 Source: youtube.com
Source: youtube.com
If you need to maintain. So multiply 1946 by 5844 and you get 11372424grams dont know how far. Herein how many mEq are in a mg of sodium chloride. For our practice problem wel. This compound is also known as Sodium Chloride.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of NaCl or grams. It is made up of sodium ions Na and chloride ions Cl. You can view more details on each measurement unit. Molecular weight of NaCl or mol. How do you find the moles of each element in NaCl.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams in 1 mole of nacl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






