Your How many grams in 1 mole of h2o images are available. How many grams in 1 mole of h2o are a topic that is being searched for and liked by netizens now. You can Get the How many grams in 1 mole of h2o files here. Download all free photos.
If you’re looking for how many grams in 1 mole of h2o images information related to the how many grams in 1 mole of h2o topic, you have come to the ideal blog. Our website always gives you hints for viewing the highest quality video and picture content, please kindly surf and locate more enlightening video content and graphics that fit your interests.
How Many Grams In 1 Mole Of H2o. In respect to this how do you find the moles of water in a hydrate. Molecular weight of Water or grams. So there will be a total of 602 1023 2 12 1024 hydrogen atoms. Divide the mass of water by the molar mass of water to get moles of water.
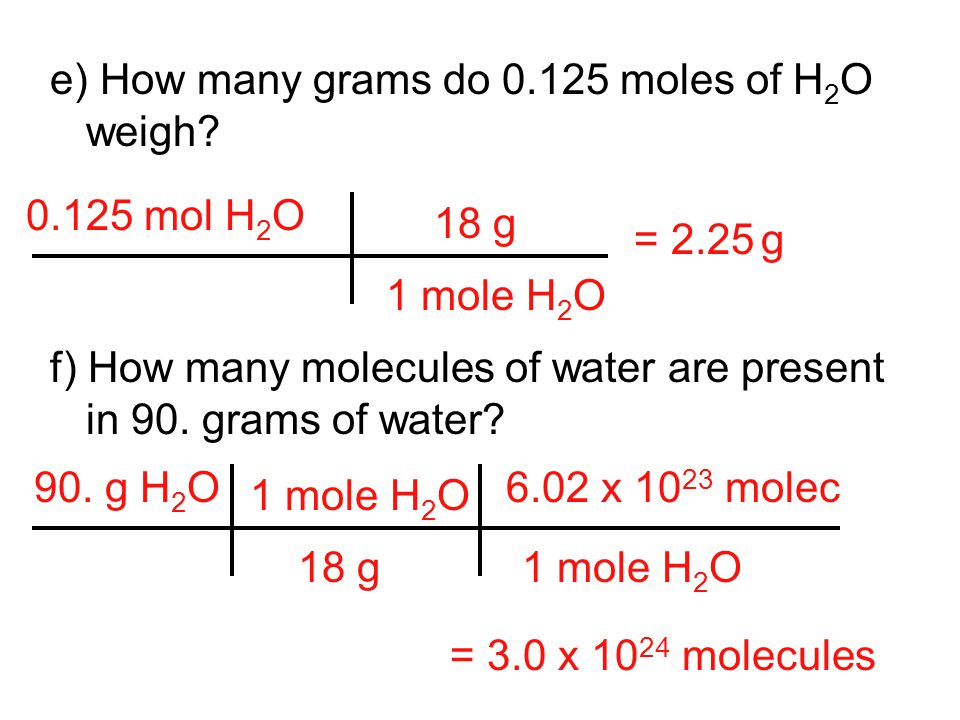 Chemical Quantities Chapter 7 10 Ppt Video Online Download From slideplayer.com
Chemical Quantities Chapter 7 10 Ppt Video Online Download From slideplayer.com
Its easier to work with grams so convert the mass. Molecular weight of CuSO4 5H2O or grams The SI base unit for amount of substance is the mole. Water has a molar mass of 1801528 gmol 18 gmol. 37 moles Na 2 O x 62 g Na 2 O 230 g Na 2 O 1 mole Na 2 O 3 How many atoms are in 14 moles of cadmium. 1 mole is equal to 1 moles H20 or 201588 grams. 54 g H2O 1 mol H2O 180152 g H2O 6022 1023 molecules 1 mol H2O.
1 mole is equal to 1 moles H20 or 201588 grams.
The number of moles of water is calculated as. 1 mole is equal to 1 moles CuSO4 5H2O or 249685 grams. Molecular weight of CuSO4 5H2O or grams The SI base unit for amount of substance is the mole. 54 g H2O 1 mol H2O 180152 g H2O 6022 1023 molecules 1 mol H2O. You can view more details on each measurement unit. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994.
 Source: clutchprep.com
Source: clutchprep.com
Molesn mass molar massn. Note that rounding errors may occur so always check the results. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles H2O or 1801528 grams. 1 mole is equal to 1 moles CuSO4 5H2O or 249685 grams.
 Source: youtube.com
Source: youtube.com
Note that rounding errors may occur so always check the results. Therefore water would weigh. We assume you are converting between moles CuSO4 5H2O and gram. Thus one mole of hydrogen has a mass of 1 gram. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams.
 Source: youtube.com
Source: youtube.com
One mole of water H2O has a mass of 18 grams. Note that rounding errors may occur so always check the results. 1 How many moles are in 400 grams of water. Use this page to learn how to convert between moles Water and gram. 1 grams H2O is equal to 0.
 Source: socratic.org
Source: socratic.org
Note that rounding errors may occur so always check the results. 1 mole is equal to 1 moles H2O or 1801528 grams. A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. 1 How many moles are in 400 grams of water. 2 hydrogen x 1g gram atomic mass of hydrogen 1 oxygen x 16g gram atomic mass of oxygen Therefore 1 mole of water contains 18grams.
 Source: slideplayer.com
Source: slideplayer.com
So the total no. The number of moles of water is calculated as. How many grams H2O in 1 molThe answer is 1801528. The molecular weight of H2O is 18g i. Note that rounding errors may occur so always check the results.
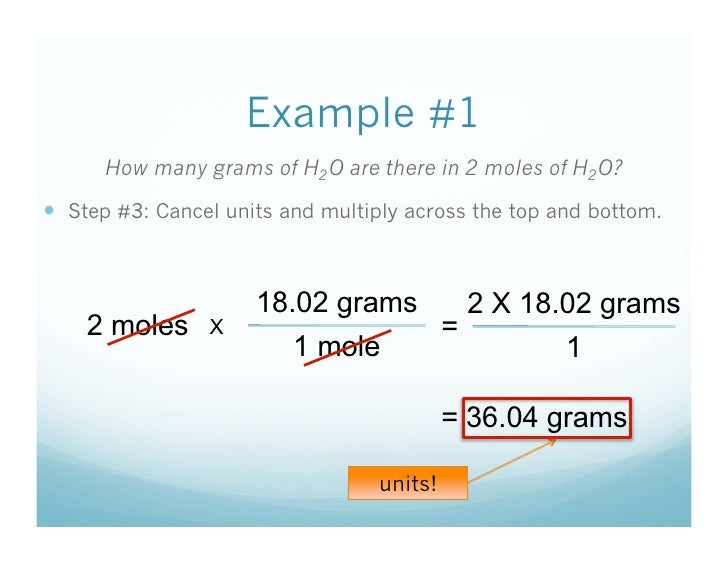 Source: pt.slideshare.net
Source: pt.slideshare.net
This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. It is equal to Avogadros number NA namely 6022 x10 23If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. We are given 1250 g of H2O. You can view more details on each measurement unit.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. Avogadros number is the conversion factor from atomic mass units AMU to grams 1 gram 602 1023 AMU. 54 g H2O 1 mol H2O 180152 g H2O 6022 1023 molecules 1 mol H2O. Therefore water would weigh. Use this page to learn how to convert between moles H2O and gram.
 Source: sites.google.com
Source: sites.google.com
In one mole of water there will exist approximately 602 1023 water molecules. The technique used can be applied to any mole to gram conversion. The SI base unit for amount of substance is the mole. Thus one mole of hydrogen has a mass of 1 gram. Therefore water would weigh.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass of H2O 180 g mol. 37 moles Na 2 O x 62 g Na 2 O 230 g Na 2 O 1 mole Na 2 O 3 How many atoms are in 14 moles of cadmium. 1 grams H2O is equal to 0. 1 How many moles are in 400 grams of water. Use this page to learn how to convert between moles 10 H2O and gram.
 Source: slideserve.com
Source: slideserve.com
1 How many moles are in 400 grams of water. 37 moles Na 2 O x 62 g Na 2 O 230 g Na 2 O 1 mole Na 2 O 3 How many atoms are in 14 moles of cadmium. Therefore water would weigh. A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. 1 mole of any substance contains 60231023 molecules.
 Source: slideplayer.com
Source: slideplayer.com
2314 mole Cd x 6022 x 10 atoms Cd 84 x 1023 atoms Cd 1 mole Cd 4 How many moles are. In this video well learn to convert moles of H2O to grams. Click to see full answer. 1801528 grams of water per mole of water. 1 mole is equal to 1 moles Water or 1801528 grams.
 Source: slideplayer.com
Source: slideplayer.com
1 mole is equal to 1 moles Water or 1801528 grams. 260 g H2 O 1800 gmol H2 O 0144 moles H2 O. We assume you are converting between moles CuSO4 5H2O and gram. A T chart is useful for this kind of problem. Note that rounding errors may occur so always check the results.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between moles CuSO4 5H2O and gram. Lets calculate the gram molecular mass of water. Note that rounding errors may occur so always check the results. Type in your own numbers in the form to convert the units. 260 g H2 O 1800 gmol H2 O 0144 moles H2 O.
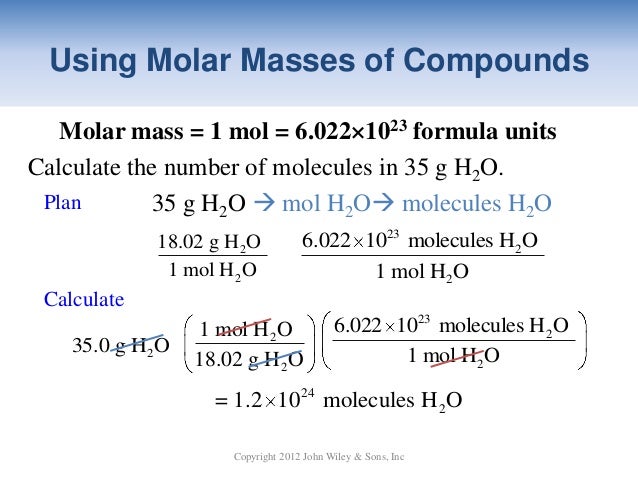 Source: slideshare.net
Source: slideshare.net
So the total no. 1 mole is equal to 1 moles CuSO4 5H2O or 249685 grams. Thus one mole of hydrogen has a mass of 1 gram. 1801528 grams of water per mole of water. In this video well learn to convert moles of H2O to grams.
 Source: slideplayer.com
Source: slideplayer.com
In this video well learn to convert moles of H2O to grams. The technique used can be applied to any mole to gram conversion. Use this page to learn how to convert between moles Water and gram. Molecular weight of CuSO4 5H2O or grams The SI base unit for amount of substance is the mole. Click to see full answer.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams in 1 mole of h2o by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






