Your How many grams in 1 mole of aluminum chloride images are ready in this website. How many grams in 1 mole of aluminum chloride are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams in 1 mole of aluminum chloride files here. Get all free photos.
If you’re looking for how many grams in 1 mole of aluminum chloride pictures information linked to the how many grams in 1 mole of aluminum chloride topic, you have come to the ideal blog. Our website always gives you hints for viewing the highest quality video and image content, please kindly hunt and find more enlightening video articles and graphics that fit your interests.
How Many Grams In 1 Mole Of Aluminum Chloride. G of silver nitrate when it is mixed with an excess of sodium chloride. How many grams Aluminium Chloride in 1 mol. 2Al s 3Cl2 g 2AlCl3 s 2 mol Al react with 3 mol Cl2 to produce 2 mol AlCl3. In the reaction of silver nitrate with sodium chloride how many grams of silver chloride will be produced from 100.
 Christmas Chemistry Activity Chemistry Activities Chemistry Chemistry For Kids From pinterest.com
Christmas Chemistry Activity Chemistry Activities Chemistry Chemistry For Kids From pinterest.com
1 mol Al 270 g Al. 270 g Al 1 mol Al 612 g Al e. We assume youre changing between grams aluminum chloride and mole. The molecular formula for Calcium Chloride is CaCl2 ie. The equation for the reaction is below. 1 grams Ammonium Chloride 001869457292809 mole using the molecular weight calculator and the molar mass of NH4Cl.
Mol AlCl 3 17 g Al.
The SI base unit for amount of substance is the mole. 1 mol Al 270 g Al. 1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams. The SI base unit for amount of substance is the mole. Molecular weight of aluminum chloride or grams. We assume you are converting between grams Aluminium Chloride and mole.
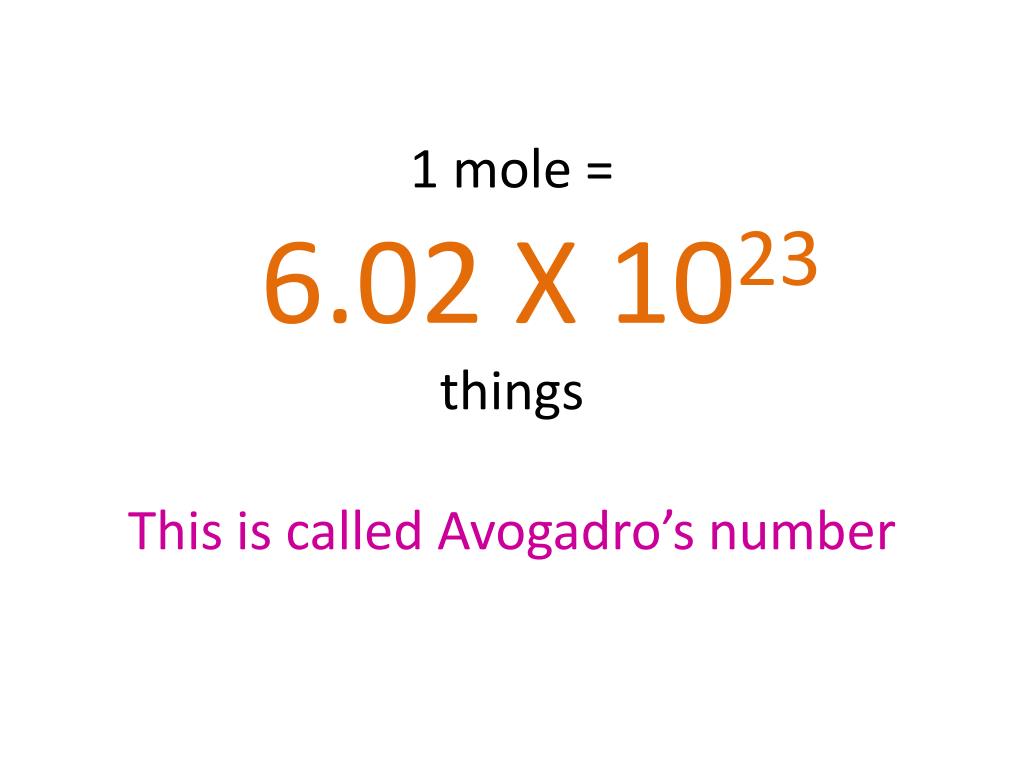 Source: slideserve.com
Source: slideserve.com
The molecular weight of AlCl3 is 13334 gmole. How many grams Aluminium Chloride in 1 mol. This compound is also called Aluminium Chloride. How many chloride ions cl 1 are present in 836 g fecl3 moles of chloride ions in aluminum chloride aluminum chloride formula what is the chloride ion concentration in a solution that is 0255 m in aluminum chloride how many moles of chloride ions are in 02550 g of aluminum chloride how many chloride ions are there in 2000 grams of ccl4. G of silver nitrate when it is mixed with an excess of sodium chloride.
 Source: youtube.com
Source: youtube.com
2Al s 3Cl2 g 2AlCl3 s 2 mol Al react with 3 mol Cl2 to produce 2 mol AlCl3. Moles massrmm 10g 2698335453 0075 moles Since the equation states that for every 3 molecules of aluminum chloride you produce 2 molecules of chlorine by cross-multiplying the amount of moles you obtain 01125 moles of chlorine. What is mole of aluminum chloride. 000191 moles AlCl3 X 3 chloridesmole of AlCl3 000573 moles of chloride. 1 mole is equal to 1 moles Aluminium Chloride or 133340538 grams.
 Source: slideplayer.com
Source: slideplayer.com
1 mole of aluminium 27g 60231023 noof atoms 27g of aluminium contains 60231023 noof atoms Therefore10g602310231027 2231023 noof. This compound is also called Aluminium Chloride. What number of grams are in a single mole of aluminum chloride. The molecular formula for Calcium Chloride is CaCl2 ie. Each mole of the salt will contain one mole of Calcium and two moles of Chlorine.
 Source: studylib.net
Source: studylib.net
AgNO3aq NaClaq AgCls NaNO3aq A 1079 g B 1699 g C 844 g D 0589 g E 589 g. 1 mole is equal to 1 moles aluminum chloride or 133340538 grams. One may also ask how many moles of chloride ions are there in 2 moles of calcium chloride. 1 mole is equal to 1 moles MgCl2 or 95211 grams. How many grams of aluminum will react with 34 moles of chlorine.
 Source: slideplayer.com
Source: slideplayer.com
G Al 34 mol Cl 2. 1 mole is equal to 1 moles MgCl2 or 95211 grams. The SI base unit for amount of substance is the mole. In the reaction of silver nitrate with sodium chloride how many grams of silver chloride will be produced from 100. M CuCl2 1 63546 gmol Cu 2 3545 gmol Cl 13445 gmol n 405 g CuCl213445 gmol 00301 mol CuCl2 In order to determine which reactant is in excess you need to calculate the amount of one product each reactant can produce.
 Source: slideplayer.com
Source: slideplayer.com
The molecular formula for Calcium Chloride is CaCl2 ie. AgNO3aq NaClaq AgCls NaNO3aq A 1079 g B 1699 g C 844 g D 0589 g E 589 g. How many grams are in 300 moles of aluminum. The molecular formula for aluminum chloride is AlCl3. This compound is also known as Magnesium Chloride.
 Source: pinterest.com
Source: pinterest.com
2Al s 3Cl2 g 2AlCl3 s 2 mol Al react with 3 mol Cl2 to produce 2 mol AlCl3. The SI base unit for amount of substance is the mole. Each mole of the salt will contain one mole of Calcium and two moles of Chlorine. This will react with 044432 0666 mol Cl2. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams. Now calcium chloride has a molar mass of approximately 111 g mol1 which means that every mole of calcium chloride has a mass of 111 g. We assume youre changing between grams aluminum chloride and mole. This is a stoichiometry problem. 1 mol AlCl 3 1335 g AlCl 3.
 Source: studylib.net
Source: studylib.net
This compound is also called Aluminium Chloride. Note that rounding errors may occur so always check the results. Answer 1 of 3. The equation for the reaction is below. The SI base unit for quantity of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
Subsequently theres 74996 mols of Al. One may also ask how many moles of chloride ions are there in 2 moles of calcium chloride. G Al 34 mol Cl 2. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2. The SI base unit for quantity of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
000191 moles AlCl3 X 3 chloridesmole of AlCl3 000573 moles of chloride. The answer is 133340538. Each mole of the salt will contain one mole of Calcium and two moles of Chlorine. How many grams are in 300 moles of aluminum. Each mole of the salt will contain one mole of Calcium.
 Source: slideplayer.com
Source: slideplayer.com
This compound is also known as Magnesium Chloride. How many grams are in 300 moles of aluminum. The SI base unit for quantity of substance is the mole. This is a stoichiometry problem. Subsequently theres 74996 mols of Al.
 Source: slideplayer.com
Source: slideplayer.com
Now calcium chloride has a molar mass of approximately 111 g mol1 which means that every mole of calcium chloride has a mass of 111 g. What is mole of aluminum chloride. Grams AlCl3 You can solve problems like this more quickly be multiplying the given quantity by a. The molecular weight of AlCl3 is 13334 gmole. 1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams.
 Source: studylib.net
Source: studylib.net
The molecular weight of AlCl3 is 13334 gmole. This is a stoichiometry problem. The molecular formula for Aluminium Chloride is AlCl3. 1 mol AlCl 3 1335 g AlCl 3. Molecular weight of aluminum chloride or grams.
 Source: studylib.net
Source: studylib.net
How many moles are in AlCl3. Do a quick conversion. Consequently how many moles of chloride ions are there in 2 moles of calcium chloride. Ol Cl 2 2 mol AlCl 3 0138 mol Cl 2 d. We assume you are converting between grams aluminum chloride and mole.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams in 1 mole of aluminum chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.