Your How many grams does 1 mole of oxygen weigh images are ready in this website. How many grams does 1 mole of oxygen weigh are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams does 1 mole of oxygen weigh files here. Get all royalty-free photos and vectors.
If you’re searching for how many grams does 1 mole of oxygen weigh pictures information connected with to the how many grams does 1 mole of oxygen weigh keyword, you have visit the right blog. Our website always gives you hints for viewing the highest quality video and image content, please kindly surf and locate more enlightening video content and images that fit your interests.
How Many Grams Does 1 Mole Of Oxygen Weigh. This means that 1 MOLE of hydrogen atoms will weigh 1008 grams. In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³ or 000082601 ounce per cubic inch ozinch³. Burning one mole of octane 114 grams therefore would produce eight moles of CO2 with a weight of 352 grams 8 x 44. 7090 g 7090 gmol.
 The Mole A Mole Mol Is A Unit Of Measurement Used For Counting Very Small Things Atoms Molecules Etc A Mole Of Something Is Similar To A Dozen Ppt Download From slideplayer.com
The Mole A Mole Mol Is A Unit Of Measurement Used For Counting Very Small Things Atoms Molecules Etc A Mole Of Something Is Similar To A Dozen Ppt Download From slideplayer.com
1 oz 12 12 oz 1 dozen eggs. How many moles is this. 1008 gramsmole Hydrogen 1 mole6022x10 23 atoms 167 x 10-24 grams. Oxygen has a molar mass approximately 1600 g mol. One gram atom of oxygen is defined as the atoms present in one gram of oxygen atom. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams.
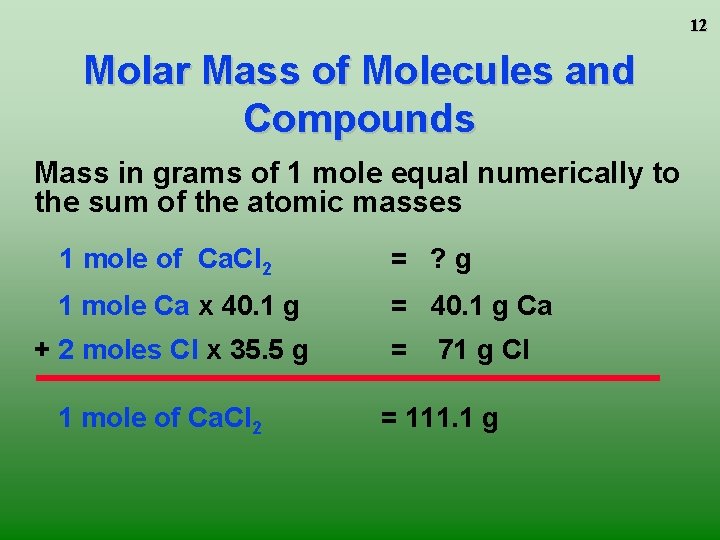
The weight of CO2 is 44 grams per mole 1 x 12 gramsmole for the carbon and 2 x 16 gramsmole for the oxygen atoms.
U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. 1 mole is equal to 1 moles Oxygen or 159994 grams. The mass of 025 mole of sodium is. At 0C 32F or 27315K at standard atmospheric pressure. Mass of one mole. X 1600 you get the number of moles.
 Source: pinterest.com
Source: pinterest.com
Write the correct formula for calcium acetate and then answer 23 - 25 based on it. I mole of Oxygen O weighs about 16gmol1But if you are talking about Oxygen gas O2 its about 32gmol-1. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. Atoms in 05 moles of copper. Use this page to learn how to convert between moles Oxygen and gram.
 Source: slideplayer.com
Source: slideplayer.com
Likewise a mol of atoms has a different weight depending on what atom youre talking about. What is the mass of exactly one mole of calcium acetate. Write the correct formula for calcium acetate and then answer 23 - 25 based on it. A dozen donuts would weigh about 24 ounces and a dozen cornflakes would weigh about 6 grams. Mass of one mole.
 Source: slideplayer.com
Source: slideplayer.com
Divide the amount you have by the molar weight to obtain the number of moles in the sample. Molar mass of oxygen 162gm 32gm. 1 mole is equal to 1 moles Oxygen or 159994 grams. Molar mass of O 159994 gmol. G Mass of one mole.
 Source: slideplayer.com
Source: slideplayer.com
One gram atom means the mass of one mole of an element equal in grams to the atomic weight. Oxygen gas is diatomic meaning two atoms of 16 gmol each so 1 mole of O2 has a mass of 32 grams. X 1600 you get the number of moles. Since the unified atomic mass unit symbol. Oxygen molecular weight.
 Source: slideplayer.com
Source: slideplayer.com
Mass of one mole. I mole of Oxygen O weighs about 16gmol1But if you are talking about Oxygen gas O2 its about 32gmol-1. X 1600 you get the number of moles. 5585 g 5585 gmol. Atomic mass is the number of grams per mole of the element.
 Source: youtube.com
Source: youtube.com
Atomic weight of oxygen is 15999 so a mole of oxygen atoms ie. 7090 g 7090 gmol. I mole of Oxygen O weighs about 16gmol1But if you are talking about Oxygen gas O2 its about 32gmol-1. X 1600 you get the number of moles. What is the mass of exactly one mole of calcium acetate.
 Source: slideplayer.com
Source: slideplayer.com
Click to see full answer. These numbers are either in atomic mass units amu or in grams per mole of atoms. Type in your own numbers in the form to convert the units. Molar mass of oxygen 162gm 32gm. Molar mass of O 159994 gmol.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Mass of 1 mole of any molecule is equal to its molar mass. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. 1008 gramsmole Hydrogen 1 mole6022x10 23 atoms 167 x 10-24 grams. Mass of 1 mole of any molecule is equal to its molar mass. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie.
 Source: slidetodoc.com
Source: slidetodoc.com
Mass of 1 mole of any molecule is equal to its molar mass. Mass of one mole. If I wanted to know how much a dozen eggs weighed I could take the average weight for one egg and multiply by 12. How many moles are contained in 158 g of the substance in 23. The weight of CO2 is 44 grams per mole 1 x 12 gramsmole for the carbon and 2 x 16 gramsmole for the oxygen atoms.
 Source: slideplayer.com
Source: slideplayer.com
Therefore the mole is a unit for that physical quantity. Oxygen has a molar mass approximately 1600 g mol. Therefore 256 g of oxygen O2 has 963x 1024 atoms of oxygens. They do not affect the number of significant figures. Atoms in 05 moles of copper.
 Source: slideplayer.com
Source: slideplayer.com
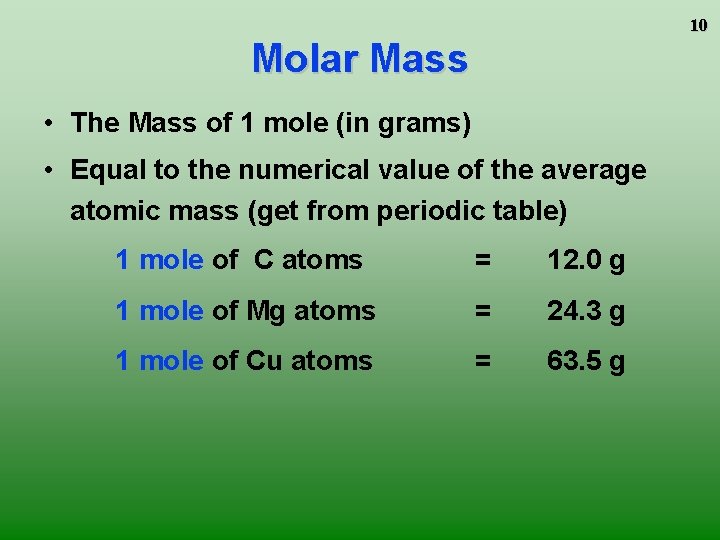
From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. These numbers are either in atomic mass units amu or in grams per mole of atoms. 7090 g 7090 gmol. Oxygen molecular weight. Note that rounding errors may occur so always check the results.
 Source: slideplayer.com
Source: slideplayer.com
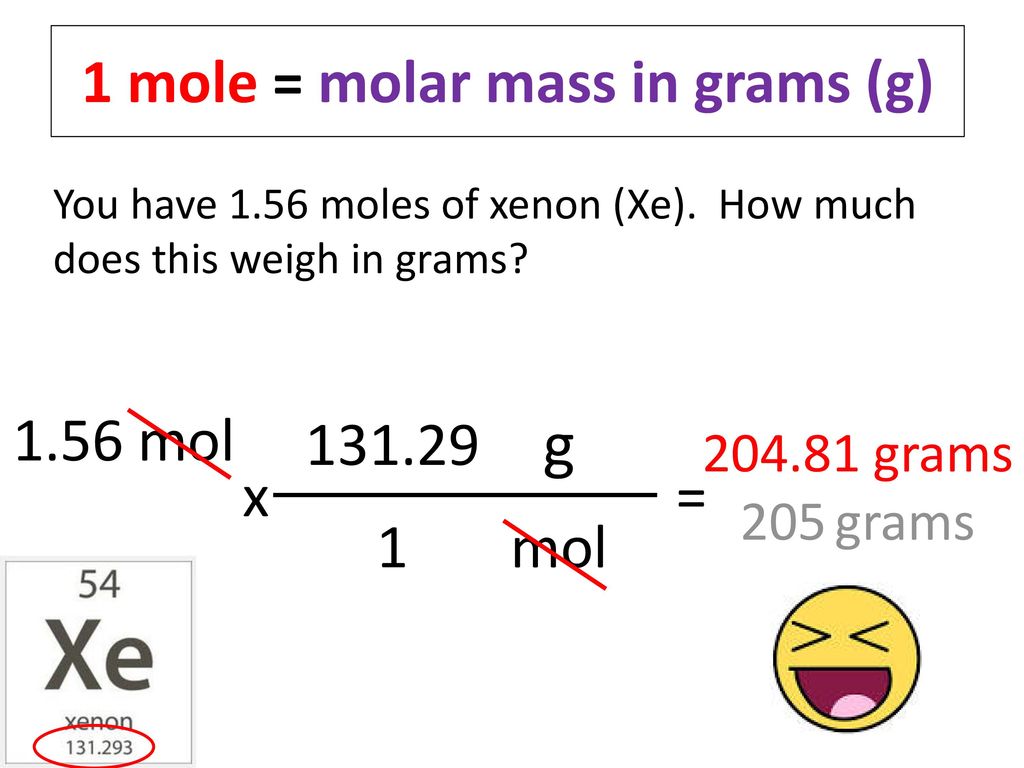
Using the periodic table complete the following. 7090 g 7090 gmol. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. The mass of 0100 mole of neon is 2018. Therefore the mole is a unit for that physical quantity.
 Source: slidetodoc.com
Source: slidetodoc.com
24 grams 32 gramsmol 075 moles. The weight of CO2 is 44 grams per mole 1 x 12 gramsmole for the carbon and 2 x 16 gramsmole for the oxygen atoms. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. 4 rows 1 10 -6. If I wanted to know how much a dozen eggs weighed I could take the average weight for one egg and multiply by 12.
 Source: slideplayer.com
Source: slideplayer.com
Oxygen has a molar mass approximately 1600 g mol. Density of oxygen is equal to 1429 kgm³. Thus mass of 1 mole of oxygen is 32gm. Using the periodic table complete the following. How many moles are contained in 158 g of the substance in 23.
 Source: toppr.com
Source: toppr.com
How many moles is this. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 10 23 atoms of oxygen. G Mass of one mole. Density of oxygen is equal to 1429 kgm³.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams does 1 mole of oxygen weigh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.