Your How many grams does 1 mole of glucose c6h12o6 weight images are ready in this website. How many grams does 1 mole of glucose c6h12o6 weight are a topic that is being searched for and liked by netizens today. You can Download the How many grams does 1 mole of glucose c6h12o6 weight files here. Download all royalty-free images.
If you’re looking for how many grams does 1 mole of glucose c6h12o6 weight pictures information linked to the how many grams does 1 mole of glucose c6h12o6 weight interest, you have come to the right blog. Our website frequently provides you with suggestions for seeking the highest quality video and image content, please kindly surf and find more informative video articles and graphics that match your interests.
How Many Grams Does 1 Mole Of Glucose C6h12o6 Weight. 1 mole is equal to 1 moles C6H12O6 or 18015588 grams. You can view more details on each measurement unit. The SI base unit for amount of substance is the mole. Mole Continue reading.
 Calculate The No Of Moles In 0 72 Grams Of Glucose Youtube From youtube.com
Calculate The No Of Moles In 0 72 Grams Of Glucose Youtube From youtube.com
The SI base unit for amount of substance is the mole. 1201076 10079412 1599946. You can view more details on each measurement unit. M C6H12O6 10 mol C6H12O6 180156 g C6H12O6mol C6H12O6 180156 g C6H12O6 2000 g C6H12O6 to one significant figure. Likewise to get a mole of glucose we need 6 moles of carbon 12 moles of hydrogen and 6 moles of oxygen. This compound is also known as Glucose or Fructose or Galactose.
Answer 1 of 2.
1 grams Glucose is equal to 0. From the chemical structure of glucose nitrate one mole is 315 grams the molar mass or molecular weight is 31 Continue reading. 1 mole is equal to 1 moles Glucose or 18015588 grams. We assume you are converting between grams Glucose and mole. Convert grams C6H12O6 to moles or moles C6H12O6 to grams. This compound is also known as Glucose or Fructose or Galactose.
 Source: in.pinterest.com
Source: in.pinterest.com
Molecular weight of C6H12O6 or grams. For our practice problem we. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. So for every 1-carbon atom there will be 2-hydrogen atoms. Molar mass of C6H12O6 18015588 gmol.
 Source:
Source:
Mole Continue reading. 1 mole is equal to 1 moles C6H12O6 or 18015588 grams. So for every 1-carbon atom there will be 2-hydrogen atoms. 1 grams Glucose is equal to 0. Mole Continue reading.
 Source: youtube.com
Source: youtube.com
From the chemical structure of glucose nitrate one mole is 315 grams the molar mass or molecular weight is 31 Continue reading. We assume you are converting between moles C6H12O6 and gram. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. The SI base unit for amount of substance is the mole. Molar mass of C6H12O6 18015588 gmol.
 Source: youtube.com
Source: youtube.com
260 x 1024 2520 x 1024 atoms. M C6H12O6 10 mol C6H12O6 180156 g C6H12O6mol C6H12O6 180156 g C6H12O6 2000 g C6H12O6 to one significant figure. Very accurate molecular weight of one mole of glucose is18015768 when the naturally abundant isotopes of these atoms. For c you would divide the number found in part b by 6023 x 1023 and in part d it is the molar mass of glucose. 1 mole is equal to 1 moles C6H12O6 or 18015588 grams.
 Source: sciencetrends.com
Source: sciencetrends.com
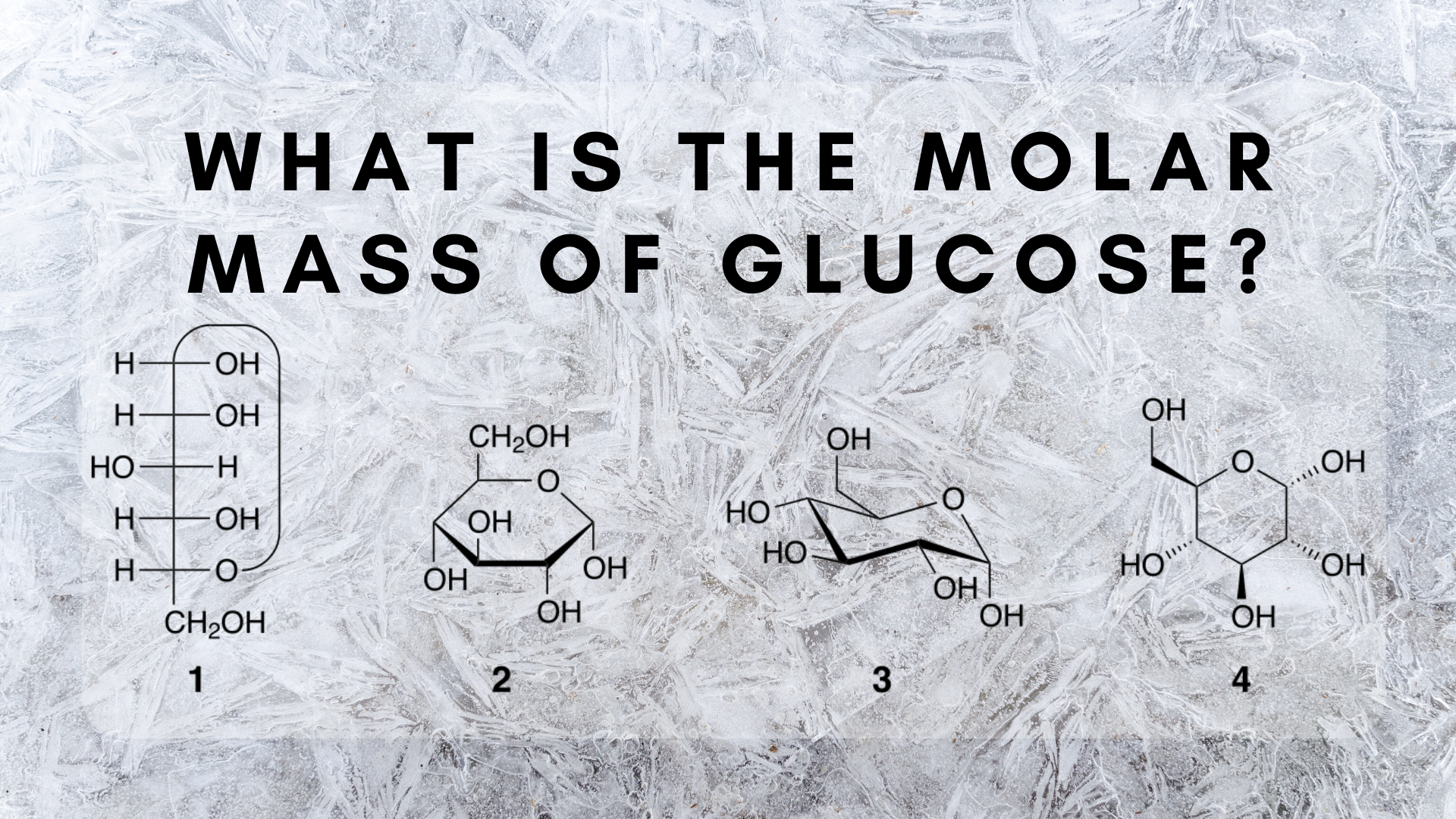
This compound is also known as Glucose or Fructose or Galactose. There are 12-Hydrogen atoms and 6-Carbon atoms in a Molecule of Glucose. We assume you are converting between grams Glucose and mole. How many moles are in C6H12O6. The technique used can be applied to any mole to gram conversion.
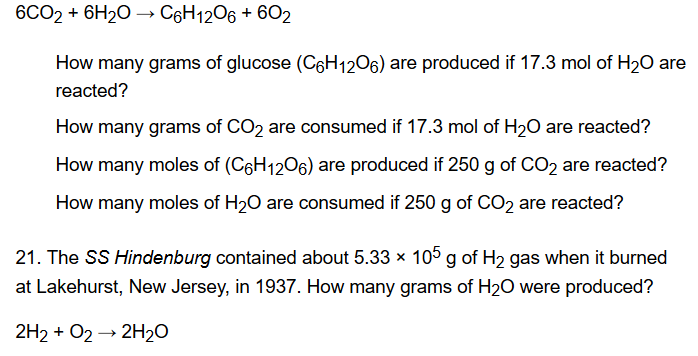 Source: chegg.com
Source: chegg.com
You can view more details on each measurement unit. Molar mass of C6H12O6 18015588 gmol. Molecular weight of C6H12O6 or grams. From the chemical structure of glucose nitrate one mole is 315 grams the molar mass or molecular weight is 31 Continue reading. You can view more details on each measurement unit.
 Source: in.pinterest.com
Source: in.pinterest.com
Type 1 diabetes misdiagnosed in many adults. How many molecules are there in 400 moles of glucose C6H12O6. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon. The technique used can be applied to any mole to gram conversion. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g.
 Source: in.pinterest.com
Source: in.pinterest.com
1 mole is equal to 1 moles Glucose or 18015588 grams. 2408 x 1024 Find the mass in grams of 200 x 1023 molecules of C10H15O called penguinone because its structure looks like a penguin. Convert grams C6H12O6 to moles or moles C6H12O6 to grams. In this video well learn to convert moles of C6H12O6 to grams. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
The technique used can be applied to any mole to gram conversion. 1 mole is equal to 1 moles Glucose or 18015588 grams. This compound is also known as Glucose or Fructose or Galactose. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon. There are 12-Hydrogen atoms and 6-Carbon atoms in a Molecule of Glucose.
 Source: youtube.com
Source: youtube.com
Likewise to get a mole of glucose we need 6 moles of carbon 12 moles of hydrogen and 6 moles of oxygen. We assume you are converting between grams Glucose and mole. Hope this helps A sample of glucose C6H12O6 contains 203x1021 atoms of carbon. How many molecules are there in 400 moles of glucose C6H12O6. Likewise to get a mole of glucose we need 6 moles of carbon 12 moles of hydrogen and 6 moles of oxygen.
 Source: in.pinterest.com
Source: in.pinterest.com
So on a per gram basis the heat released is 4950 kJ divided by 306 g or -162 kJgram. The technique used can be applied to any mole to gram conversion. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles C6H12O6 or 18015588 grams. For c you would divide the number found in part b by 6023 x 1023 and in part d it is the molar mass of glucose.
 Source: youtube.com
Source: youtube.com
Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. 1 mole is equal to 1 moles Glucose or 18015588 grams. Molecular weight of Glucose ormol The molecular formula for Glucose is C6H12O6. So for every 1-carbon atom there will be 2-hydrogen atoms. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon.

This compound is also known as Glucose or Fructose or Galactose. There are 12-Hydrogen atoms and 6-Carbon atoms in a Molecule of Glucose. For c you would divide the number found in part b by 6023 x 1023 and in part d it is the molar mass of glucose. This compound is also known as Glucose or Fructose or Galactose. To get a single glucose molecule C6H12O6 we need 6 carbon atoms 12 hydrogen atoms and finally 6 oxygen atoms.
 Source: youtube.com
Source: youtube.com
In this video well learn to convert moles of C6H12O6 to grams. Molecular weight of Glucose or grams The molecular formula for Glucose is C6H12O6. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. 260 x 1024 2520 x 1024 atoms. 2408 x 1024 Find the mass in grams of 200 x 1023 molecules of C10H15O called penguinone because its structure looks like a penguin.
 Source: youtube.com
Source: youtube.com
In this video well learn to convert moles of C6H12O6 to grams. You can view more details on each measurement unit. We assume you are converting between moles C6H12O6 and gram. You can view more details on each measurement unit. From the chemical structure of glucose nitrate one mole is 315 grams the molar mass or molecular weight is 31 Continue reading.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams does 1 mole of glucose c6h12o6 weight by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






