Your How many grams does 1 mole of fe weigh images are ready. How many grams does 1 mole of fe weigh are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams does 1 mole of fe weigh files here. Get all royalty-free images.
If you’re searching for how many grams does 1 mole of fe weigh pictures information related to the how many grams does 1 mole of fe weigh keyword, you have come to the ideal site. Our site always gives you hints for refferencing the maximum quality video and picture content, please kindly surf and locate more informative video articles and graphics that fit your interests.
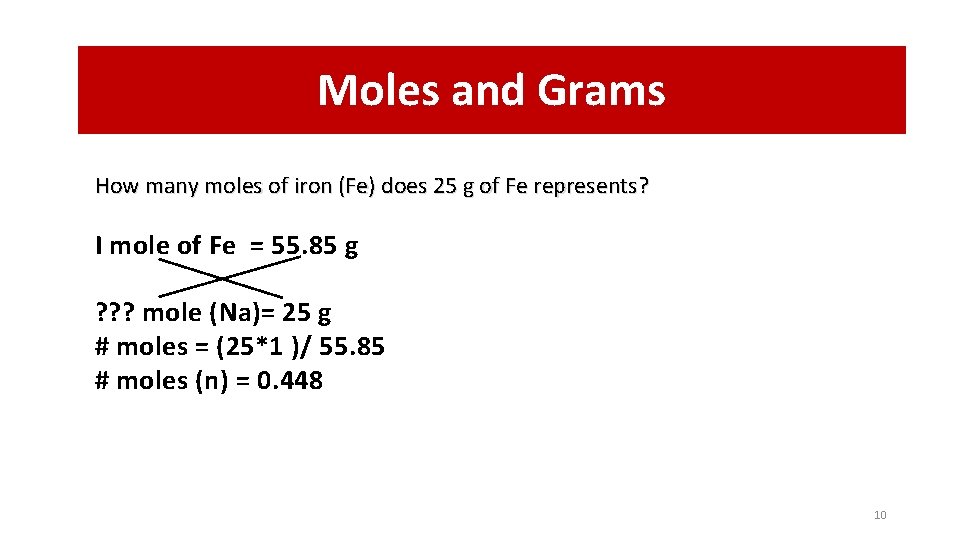
How Many Grams Does 1 Mole Of Fe Weigh. Knowing that we have 0013 moles of Fe we could now convert that into grams by knowing that one mole of Fe has a mass of 5585 grams. Therefore water would weigh. Then the mass can be calculated using the molar mass. This makes it easier to see how grams cancel leaving moles.
 Chapter 7 Chemical Quantities Mole Amount Of Pure Substance That From studylib.net
Chapter 7 Chemical Quantities Mole Amount Of Pure Substance That From studylib.net
Then the mass can be calculated using the molar mass. Grams moles atoms. 168 g Fe 1 mol Fe56 g Fe 30 mol Fe to two significant figures. How many grams of ironIII oxide is 34x1036 molecules of Fe 2 O 3. Atomic mass is the number of grams per mole of the element. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams.
1 mole is equal to 1 moles Fe or 55845 grams.
Then the mass can be calculated using the molar mass. Since molar mass is a fraction you can divide by multiplying by its reciprocal molg. Convert 9500g of iron to number of atoms in the sample. 168 g Fe 1 mol Fe56 g Fe 30 mol Fe to two significant figures. Convert grams Fe to moles or moles Fe to grams Percent composition by element. Chlorous acid HClO 2 contains what percent hydrogen by mass.
 Source: slidetodoc.com
Source: slidetodoc.com
Knowing that we have 0013 moles of Fe we could now convert that into grams by knowing that one mole of Fe has a mass of 5585 grams. Mass of 1 H atom. How many grams of ironIII oxide is 34x1036 molecules of Fe 2 O 3. 602x1023 particles 1 mol molar mass 224 L STP 6. How many molecules of ammonium chloride are in 134 L of NH 4 Cl at STP.

One molecule of a compound weighs 293 10 22 g. One mole of S atoms has a mass of 3207 g. How many oxygen atoms are there in 175 ng of Ca 3 PO 4 2. One molecule of a compound weighs 293 10 22 g. 60 mole O 2 x 2 mole Fe 2O 3 40 mole Fe 2O.
 Source: slideplayer.com
Source: slideplayer.com
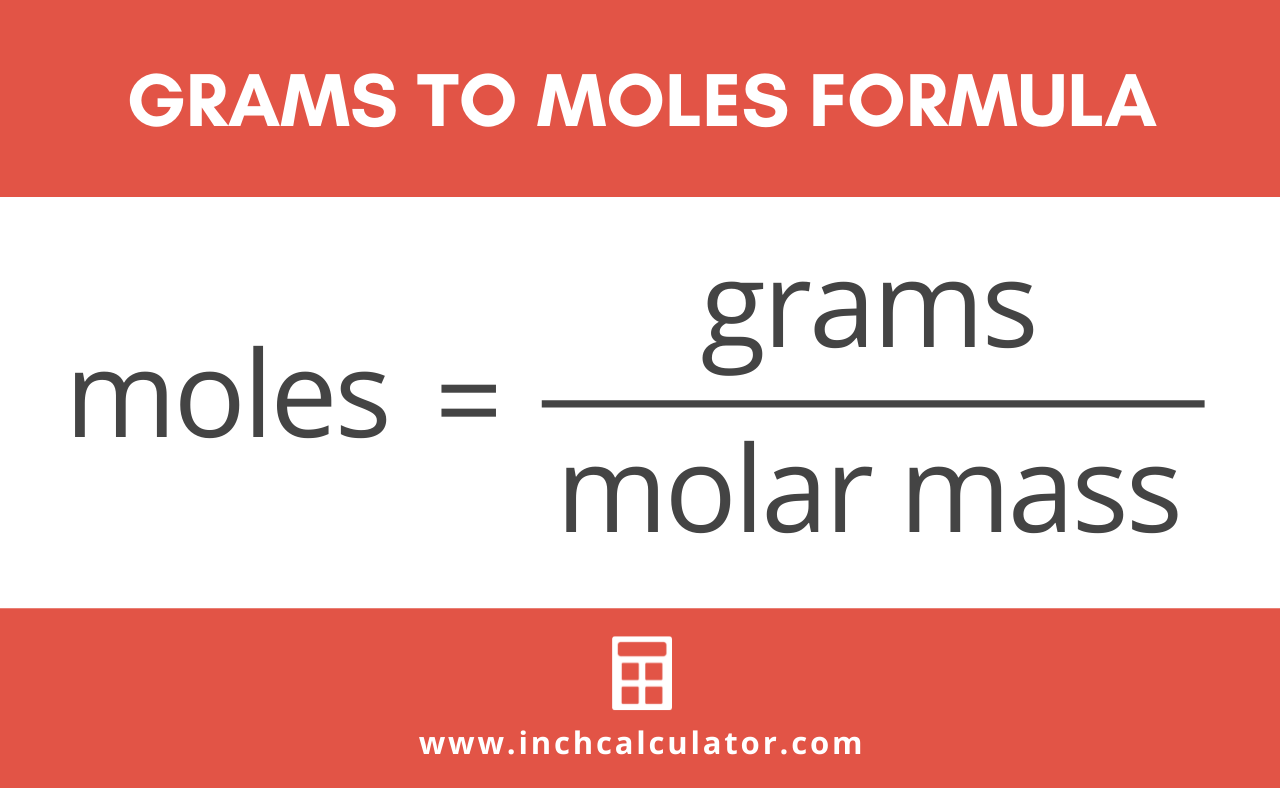
Use this page to learn how to convert between moles Fe and gram. Convert 9500g of iron to number of atoms in the sample. 602x1023 particles 1 mol molar mass 224 L STP 6. M molar mass 56 gmol According to the formula divide given mass in grams by the molar mass. Molar mass of Fe 55845 gmol.
 Source: slidetodoc.com
Source: slidetodoc.com
This makes it easier to see how grams cancel leaving moles. 60 mole O 2 x 2 mole Fe 2O 3 40 mole Fe 2O. How many oxygen atoms are there in 175 ng of Ca 3 PO 4 2. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams.
 Source: studylib.net
Source: studylib.net
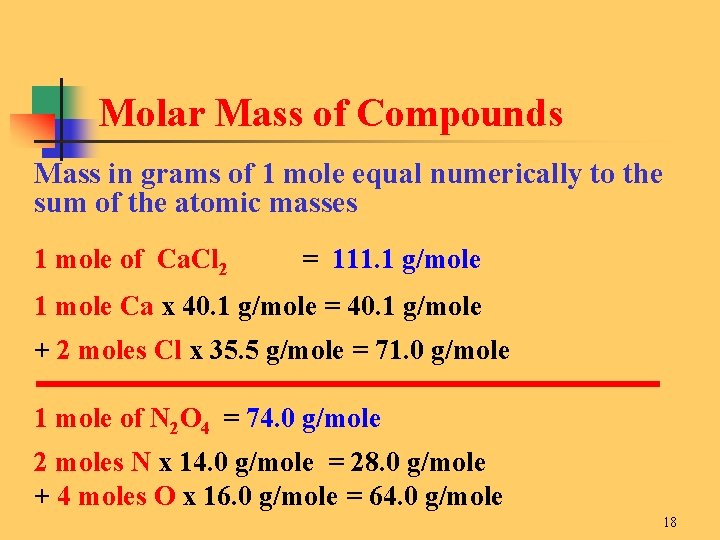
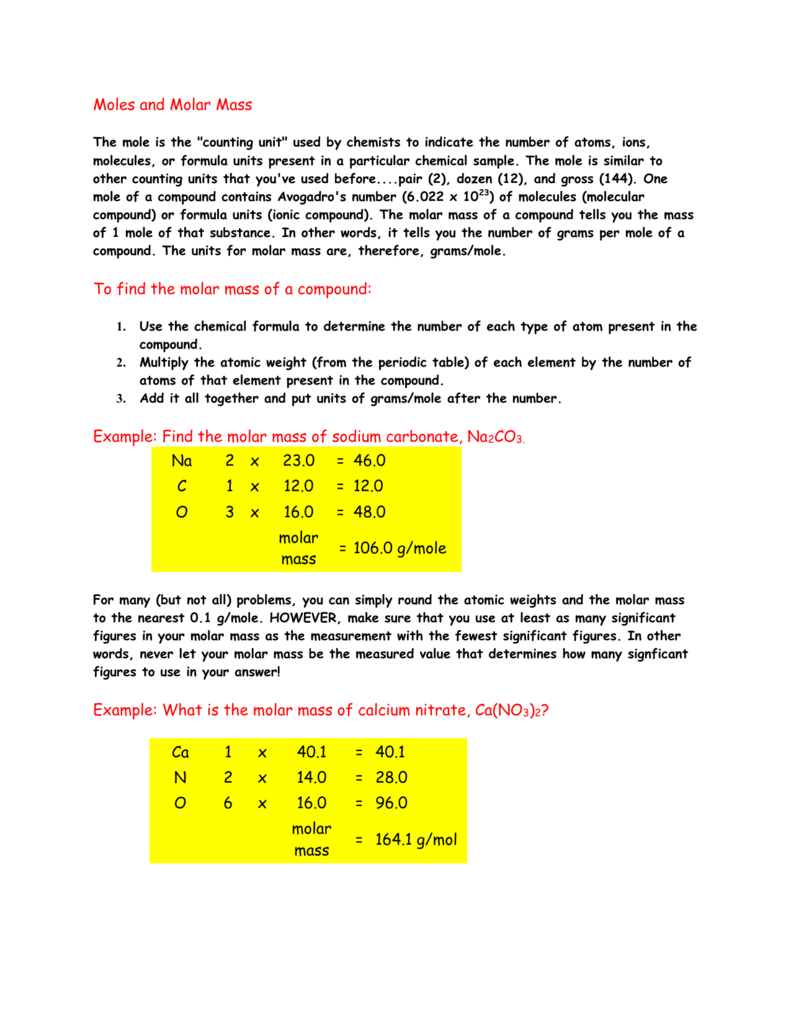
What is the molar mass of this compound. Grams Fe 2O3 moles Fe 2O3 grams Fe moles Fe Stoichiometry Chapter 4 molar mass molar mass molar ratio g Fe 50 g Fe 2O3 x 1 mol Fe 2O3 x 1597 g Fe 2O3 5585 g Fe 1 mol Fe x 4 mol Fe 2 mol Fe 2O3 35 g Fe Molar Mass of Fe 2O3 2 5585 gmole 3 160 gmole 1597 g Fe 2O3mole Stoichiometry Chapter 4 x 4 Fe 3 O 2 2 Fe 2O3. Molecular weight of Fe or grams. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5. Note that rounding errors may occur so always check the results.
 Source: youtube.com
Source: youtube.com
Thus the mass of one mole of iron is. Grams Fe 2O3 moles Fe 2O3 grams Fe moles Fe Stoichiometry Chapter 4 molar mass molar mass molar ratio g Fe 50 g Fe 2O3 x 1 mol Fe 2O3 x 1597 g Fe 2O3 5585 g Fe 1 mol Fe x 4 mol Fe 2 mol Fe 2O3 35 g Fe Molar Mass of Fe 2O3 2 5585 gmole 3 160 gmole 1597 g Fe 2O3mole Stoichiometry Chapter 4 x 4 Fe 3 O 2 2 Fe 2O3. On the other hand a dozen cans of soda would have a different weight. Atomic mass is the number of grams per mole of the element. Tap card to see definition.
 Source: studylib.net
Source: studylib.net
How many molecules of ammonium chloride are in 134 L of NH 4 Cl at STP. M molar mass 56 gmol According to the formula divide given mass in grams by the molar mass. Convert 9500g of iron to number of atoms in the sample. Since molar mass is a fraction you can divide by multiplying by its reciprocal molg. Thus the mass of one mole of iron is.
 Source: youtube.com
Source: youtube.com
How did I know how much a helium atom weighs. How many liters of carbon tetrachloride are in 43 moles of CCl 4 STP. They both contain 602 x 10 23 atoms. The molar mass of a substance is the mass of one mole of the substance. How many molecules of ammonium chloride are in 134 L of NH 4 Cl at STP.
 Source: lisbdnet.com
Source: lisbdnet.com
Note that rounding errors may occur so always check the results. 4Fes 3O 2g 2Fe 2O 3s Relationship. How many grams are in one mole of Fe. The SI base unit for amount of substance is the mole. What is the mass of 0250 moles of aluminum.
 Source: khanacademy.org
Source: khanacademy.org
9500 g Fe 1 mole 602 x 1023 atoms 10 x 1026 atoms 558g 1 mole 6. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5. Atomic mass is the number of grams per mole of the element. Convert grams Fe to moles or moles Fe to grams Percent composition by element. How did I know how much a helium atom weighs.
 Source: toppr.com
Source: toppr.com
Grams moles atoms. To obtain the mass of one iron atom divide the mass of one mole of iron atom with the number of iron atoms present in one mole. How many moles of Fe 2O 3 can form from 60 mole O 2. 12 oz 12 144 oz 1 dozen cans of soda. 0250 moles 270g 675 g Al 1 mole 7.
 Source: studylib.net
Source: studylib.net
Molecular weight of Fe or grams. With the actual numbers. How many grams of ironIII oxide is 34x1036 molecules of Fe 2 O 3. Grams Cl2 moles Cl2 moles Fe grams Fe ratio ratio ratio 750 g Cl2 1 mol Cl2 7090 g Cl2 4 mol Fe 3 mol Cl2 788 g Fe 55. Chlorous acid HClO 2 contains what percent hydrogen by mass.
 Source: slidetodoc.com
Source: slidetodoc.com
Tap again to see term. What is the molar mass of this compound. Convert 9500g of iron to number of atoms in the sample. 602x1023 particles 1 mol molar mass 224 L STP 6. 1008 amu x 1661 x10-24 gamu 1674 x10-24 g Mass of 1 mole of H atoms.
 Source: inchcalculator.com
Source: inchcalculator.com
This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. 60 mole O 2 x 2 mole Fe 2O 3 40 mole Fe 2O. Tap card to see definition. To obtain the mass of one iron atom divide the mass of one mole of iron atom with the number of iron atoms present in one mole. Atomic mass is the number of grams per mole of the element.
 Source: studylib.net
Source: studylib.net
Click again to see term. 12 oz 12 144 oz 1 dozen cans of soda. The mass m of Fe is. Grams moles atoms. We could do the same thing with atoms – for example a mol of helium.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams does 1 mole of fe weigh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






