Your How many grams are there in one mole of iron images are ready. How many grams are there in one mole of iron are a topic that is being searched for and liked by netizens today. You can Get the How many grams are there in one mole of iron files here. Find and Download all free vectors.
If you’re looking for how many grams are there in one mole of iron pictures information linked to the how many grams are there in one mole of iron interest, you have come to the right site. Our site always provides you with hints for viewing the highest quality video and image content, please kindly surf and locate more enlightening video content and images that fit your interests.
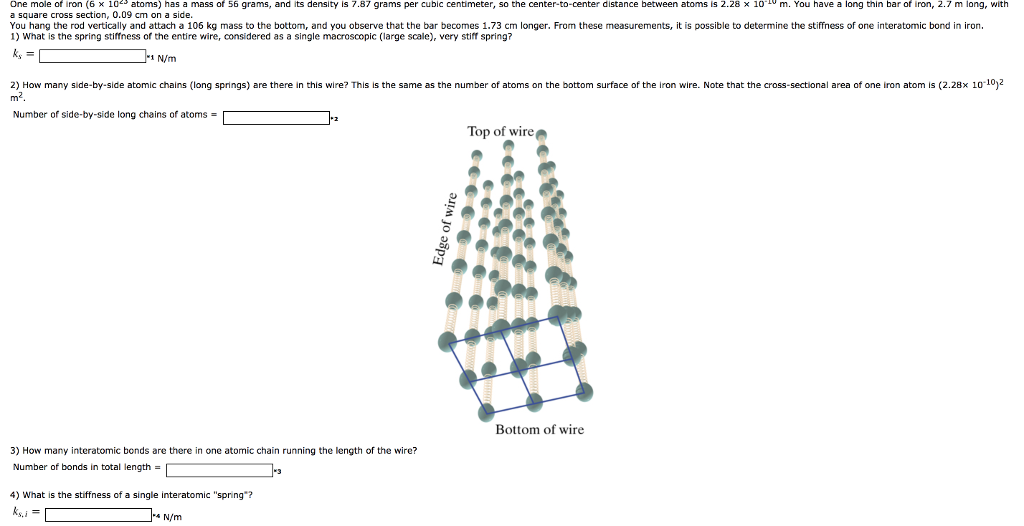
How Many Grams Are There In One Mole Of Iron. How many grams is this. 41 mol You have 132 moles of lead II nitrate. How many moles are 285 x 10 18 atoms of iron. Of Fe is 56So in one mole of Fe2O3 there are 256112 gm of Fe.
 Calculate The Number Of Iron Atoms In A Piece Of Iron Weighing 2 8 G Atomic Mass Of Iron 56 U Youtube From youtube.com
Calculate The Number Of Iron Atoms In A Piece Of Iron Weighing 2 8 G Atomic Mass Of Iron 56 U Youtube From youtube.com
Therefore 1 mole of iron will weigh 56 grams. This refers to one mole of anything eggs paperclips atoms. To convert a given mass of phosphorus to moles 5. The SI base unit for amount of substance is the mole. To obtain the mass of one iron atom divide the mass of one mole of iron atom with the number of iron atoms present in one mole. 1 10 -6.
1 mole is equal to 1 moles Iron or 55845 grams.
169 milligrams iron 1 gram1000 milligrams1 mole Fe5585 grams6022 X 10231 mole Fe 182 X 1021 atoms of iron —–. Of Fe is 56So in one mole of Fe2O3 there are 256112 gm of Fe. 1 10 -6. A 25 497 10 B 5585 C 0677 D 148 E none of the above. 1 000 000 000 000. 1008 amu x 1661 x10-24 gamu 1674 x10-24 g.
 Source: slideplayer.com
Source: slideplayer.com
3A2 2B C 2D How many moles of D can be formed from 50 mol of A2 and excess B. One mole is in the question How many moles of Na contain 145x10 21 atoms of Na. Mass of 1 H atom. How many grams are there in 21 moles of sodium. How many moles are 285 x 10 18 atoms of iron.
 Source: toppr.com
Source: toppr.com
Kilogram per mole kgmol 006. How many grams is this. 169 milligrams iron 1 gram1000 milligrams1 mole Fe5585 grams6022 X 10231 mole Fe 182 X 1021 atoms of iron —–. 0250 moles 270g 675 g Al 1 mole 7. The number 60221023 is commonly referred to as the constant of Avogadro and is often denoted by the NA symbol.
 Source: clutchprep.com
Source: clutchprep.com
How many grams is this. To convert a given mass of phosphorus to moles 5. A 25 497 10 B 5585 C 0677 D 148 E none of the above. Now gram atomic wtof Fe is 56So in one mole of Fe2O3 there are 256112 gm of Fe. The molar mass of phosphorus rounds to 3097 gmol.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between moles Iron and gram. One mole is in the question How many moles of Na contain 145x10 21 atoms of Na. 4 How many moles are there in 23 grams of phosphorus. There are ______ number of molecules atoms of NaCl in one mole of NaCl. To convert a given mass of phosphorus to moles 5.
 Source: chegg.com
Source: chegg.com
The molar mass of iron is text 56 gmole. Convert 9500g of iron to number of atoms in the sample. 1 Counting Atoms How many iron atoms are present in 300 moles of iron metal. 1 mole is equal to 1 moles IronIII Nitrate or 2418597 grams. 1 amu 1661 X 10-24g this is roughly equal to the mass of ONE proton Because the mass of one amu is so small chemists deal with a much larger number of atoms while working with chemicals.
 Source: chegg.com
Source: chegg.com
One mole of anything is 6022 X 1023 items. Hence the number of grams of sodium is present in 2 moles is 46g. 437 x 103 g How many pounds would 575 moles of magnesium hydroxide weigh0739 lb How many moles are there in 78 grams of nitrogen. 1 grams Iron is equal to 001790670606142 mole. Kilogram per mole kgmol 006.
 Source: alamy.com
Source: alamy.com
Kilogram per mole kgmol 006. 1 mol Fe 5585 g Fe 602 x 1023 atoms Fe x atoms Fe 300 mol Fe 1 mol Fe 602 x 1023 atoms Fe x mol Fe 300 mol Fe x 602 x 1023 atoms Fe 181 x 1024 atoms Fe 1 mol Fe 0174 mol S x 602 x 1023 atoms S 1 mol S 592 x 1024 atoms K x 1 mol K 602 x 1023. 15 How many moles are there in 825 grams of iron. Hence in 175moles Fe2O3 there are 1752 moles or 17556 gm of iron. At 20C 68F or 29315K at standard atmospheric pressure.
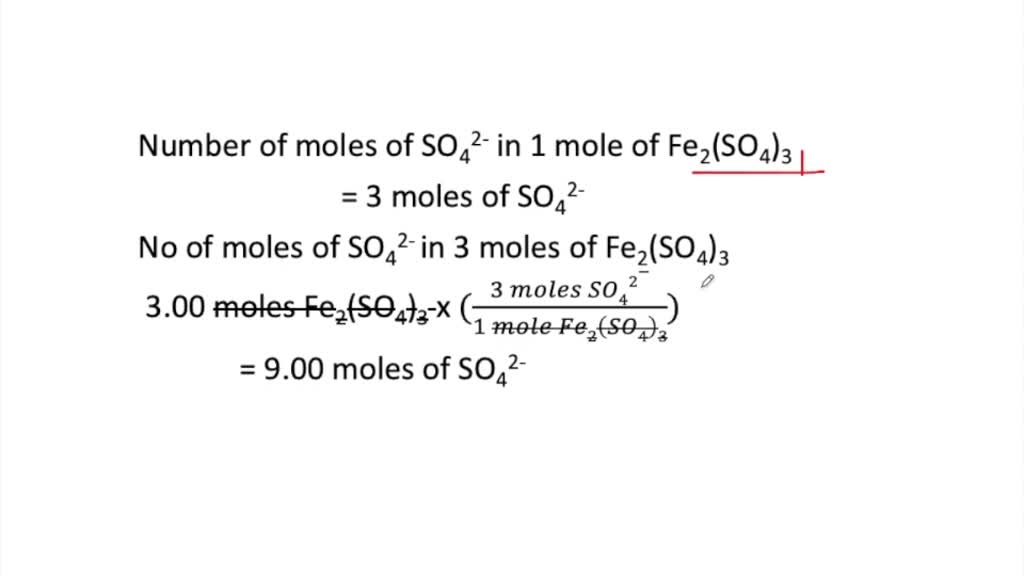 Source: numerade.com
Source: numerade.com
Therefore 1 mole of iron will weigh 56 grams. What is the mass of 0250 moles of aluminum. Mass of 1 H atom. Mass of a mole of particles mass of 1 particle x 6022 x 1023 The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams. 15 How many moles are there in 825 grams of iron.
 Source: youtube.com
Source: youtube.com
15 How many moles are there in 825 grams of iron. Use this page to learn how to convert between moles Iron and gram. One mole is defined as 6022 X 1023. 4 How many moles are there in 23 grams of phosphorus. One atom of sulfur has a mass of 3207 amu.
 Source: texasgateway.org
Source: texasgateway.org
1 mol Fe 5585 g Fe 602 x 1023 atoms Fe x atoms Fe 300 mol Fe 1 mol Fe 602 x 1023 atoms Fe x mol Fe 300 mol Fe x 602 x 1023 atoms Fe 181 x 1024 atoms Fe 1 mol Fe 0174 mol S x 602 x 1023 atoms S 1 mol S 592 x 1024 atoms K x 1 mol K 602 x 1023. Mass of 1 H atom. 437 x 103 g How many pounds would 575 moles of magnesium hydroxide weigh0739 lb How many moles are there in 78 grams of nitrogen. The molecular formula for Iron is Fe. How many moles are there in 768 grams of iron III sulfate.
 Source: mccord.cm.utexas.edu
Source: mccord.cm.utexas.edu
How many grams is equal to 348 x 1022 atoms of tin. How many moles are 285 x 10 18 atoms of iron. How many grams is equal to 348 x 1022 atoms of tin. The molar mass of iron is text 56 gmole. 15 How many moles are there in 825 grams of iron.

How many moles of copper are. Mass of substance Amount of substance in moles Number of atoms molecules or formula units of substance Convert using the molar. 437 x 103 g How many pounds would 575 moles of magnesium hydroxide weigh0739 lb How many moles are there in 78 grams of nitrogen. Hence in 175molesFe2O3 there are 1752 moles or 17556 gm of iron. One mole is in the question How many moles of Na contain 145x10 21 atoms of Na.
 Source: clutchprep.com
Source: clutchprep.com
The molecular formula for Iron is Fe. 169 milligrams iron 1 gram1000 milligrams1 mole Fe5585 grams6022 X 10231 mole Fe 182 X 1021 atoms of iron —–. 55847 g of iron 6022 1023 iron atoms 1 mol of iron 32066 g of sulfur 6022 1023 sulfur atoms 1 mol of sulfur General Plan for Converting Mass Amount and Numbers of Particles Use Avogadros number for conversion. Gram per mole gmol 5585. 169 milligrams iron 1 gram1000 milligrams1 mole Fe5585 grams6022 X 10231 mole Fe 182 X 1021 atoms of iron —–.

One atom of sulfur has a mass of 3207 amu. Iron weighs 7873 gram per cubic centimeter or 7 873 kilogram per cubic meter ie. In one mole of fe2o3 there are two moles of iron atoms. 41 mol You have 132 moles of lead II nitrate. One mole of anything is 6022 X 1023 items.
 Source: youtube.com
Source: youtube.com
192 mol How many moles are there in 12 kg of potassium dichromate. How many moles are 285 x 10 18 atoms of iron. Use this page to learn how to convert between grams Iron and mole. 41 mol You have 132 moles of lead II nitrate. How many grams is this.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are there in one mole of iron by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






