Your How many grams are in one mole of neon images are available. How many grams are in one mole of neon are a topic that is being searched for and liked by netizens today. You can Get the How many grams are in one mole of neon files here. Find and Download all royalty-free photos and vectors.
If you’re looking for how many grams are in one mole of neon images information linked to the how many grams are in one mole of neon keyword, you have pay a visit to the ideal blog. Our website always gives you hints for seeking the maximum quality video and picture content, please kindly search and locate more enlightening video content and images that match your interests.
How Many Grams Are In One Mole Of Neon. The provided mass of glycine 28 g is a bit more than one-third the molar mass 75 gmol so we would expect the computed result to be a bit greater than one-third of a mole 033 mol. A tank contains 500 moles of O 2 300 moles of neon 600 moles of H 2 S and 400 moles of argon at a total pressure of 16200 mm. A mole of aluminum is 27g because aluminums relative atomic mass is 27. Although the description of these basic units can be found in a text of any college physics the definition of a mole is important for engineering calculations.
 Solved Which Statement Is False View Available Hint S Chegg Com From chegg.com
Solved Which Statement Is False View Available Hint S Chegg Com From chegg.com
How much mass does 01 mole of neon contain. One unified atomic mass unit is approximately the mass of one nucleon either a single proton or neutron and is numerically equivalent to 1 gmol. There are many vapor pressure of water data sets available on the Internet and many of them include mmHg values. We must first evaluate R the molar gas constant. How many molecules per cubic centimeter is this. One atomic mass unit is equal to 166 x 10-24 grams.
What volume would a 0250 mole sample of H2 gas occupy if it had a which has a pressure of 170 atm and a temperature of 35 C.
Therefore one mole of iron has a weight of 55847 grams. Historically the mole was defined as the amount of substance in 12 grams of the carbon-12 isotope. Quantities Symbols and Units Quantity Symbol Unit length L. A mole of aluminum also contains 620 billion trillion. C grams D anions E milliliters 21The gram formula mass of C 7 H 16 and the gram formula mass of CaCO 3 contain approximately the same number of _____. The mole is simply a very large number 6022 xx 1023 that has a special propertyIf I have 6022 xx 1023 hydrogen atoms I have a mass of 1 gram of hydrogen atomsIf I have 6022 xx 1023 H_2 molecules I have a mass of 2 gram of hydrogen molecules.
 Source: numerade.com
Source: numerade.com
Quantities Symbols and Units Quantity Symbol Unit length L. 26018 g 202 g 20 g 2 g. C grams D anions E milliliters 21The gram formula mass of C 7 H 16 and the gram formula mass of CaCO 3 contain approximately the same number of _____. The Avogadro constant is named after the early nineteenth century Italian scientist Amedeo Avogadro. A tank contains 500 moles of O 2 300 moles of neon 600 moles of H 2 S and 400 moles of argon at a total pressure of 16200 mm.
 Source: pinterest.com
Source: pinterest.com
How much mass does 01 mole of neon contain. Electronegativity of Neon is. We must first evaluate R the molar gas constant. Here is an example of one. PV 1 atm224 L L-atm.
 Source: chegg.com
Source: chegg.com
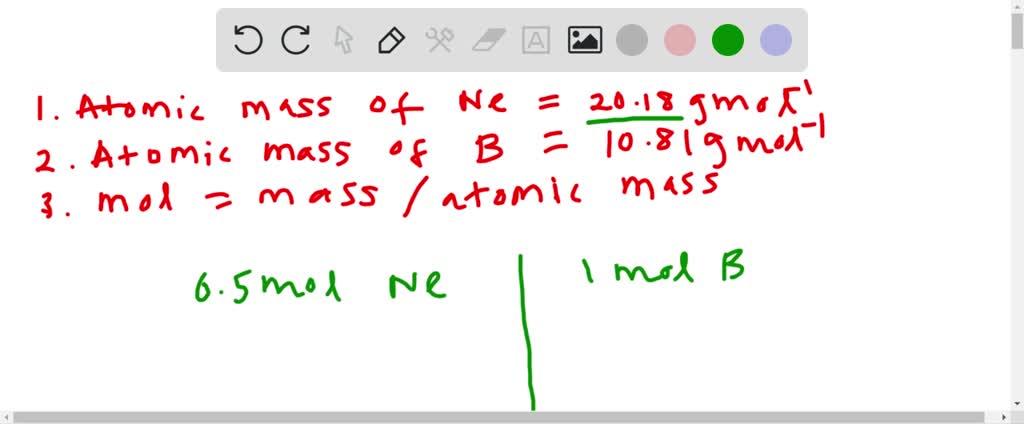
A mole of aluminum is 27g because aluminums relative atomic mass is 27. The mass of 0100 mole of neon is 2018 grams. Quantities Symbols and Units Quantity Symbol Unit length L. This answer will have 3 SF 540 g601 g00185 g0167 g. How much mass does 01 mole of neon contain.
 Source: toppr.com
Source: toppr.com
There will be as many molecules of the substances on one side of the arrow as there are molecules of the substances on the. A mole of aluminum also contains 620 billion trillion. 17 Full PDFs related to this paper. A mole of aluminum is 27g because aluminums relative atomic mass is 27. Full PDF Package Download Full PDF Package.
 Source: pinterest.com
Source: pinterest.com
4 Note that the problem says atmospheric. Solve the IDEAL GAS LAW for R and substitute known values for n P V and T. How many molecules is this. Let us assign 1 mole for the amount of neon gas and assign it to be n 1. 008206 L-atmK-mole Tabsolute temperature Kelvin Sample Problem.
 Source: numerade.com
Source: numerade.com
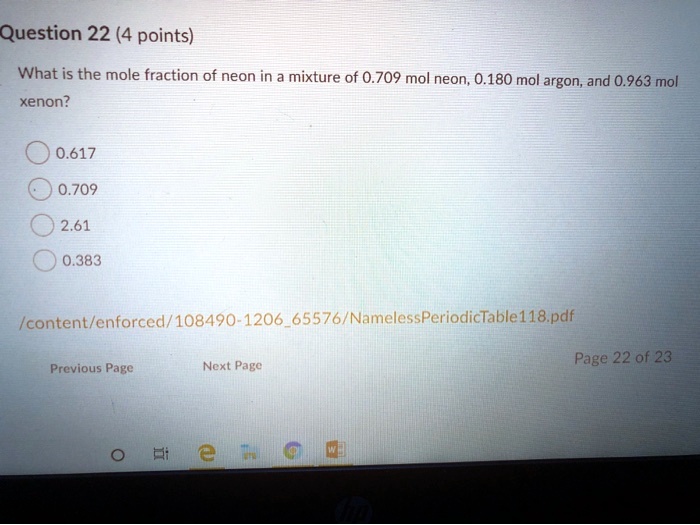
A mole is the amount of substance con-taining 6023x1023 number of particles. In chemistry and atomic physics the electron affinity of an atom or molecule is defined as. A mole of aluminum also contains 620 billion trillion. 36 If a 100 liter vessel at room temperature is evacuated to a pressure of 50 mTorr how many moles of gas are in the vessel. The mass of argon now added is exactly equal to the neon but argon has a higher gram-atomic weight molar mass than neon.
 Source: brainly.com
Source: brainly.com
Full PDF Package Download Full PDF Package. A mole of aluminum is 27g because aluminums relative atomic mass is 27. 17 Full PDFs related to this paper. Critical density grams per cubic centimetre 00696 04819 05356 09092 1103 thermal conductivity watts per metre Kelvin 01513 00491 00177 00094 00057 00036 magnetic susceptibility cgs units per mole 00000019 00000072. There are many vapor pressure of water data sets available on the Internet and many of them include mmHg values.
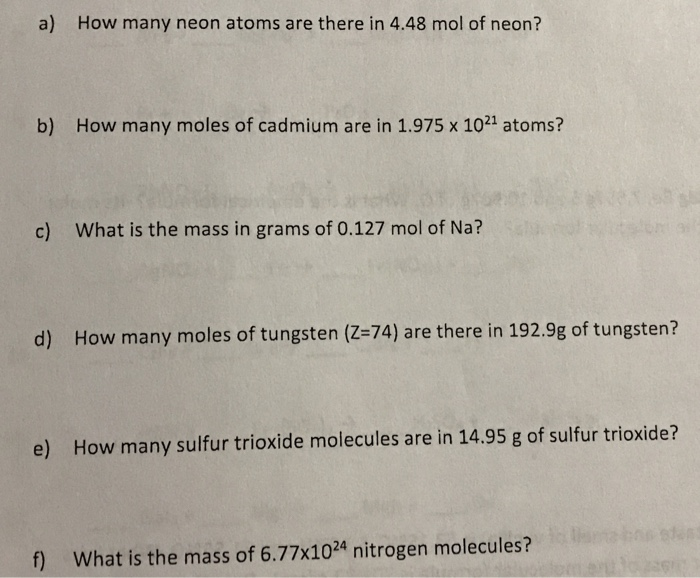 Source: chegg.com
Source: chegg.com
26018 g 202 g 20 g 2 g. The mass of 0100 mole of neon is 2018 grams. A short summary of this paper. 35453 amu which gives the molar weight of salt as 5844 grams. The change in energy in kJmole of a neutral atom or molecule in the gaseous phase when an electron is added to the atom to form a negative ion.
 Source: brainly.in
Source: brainly.in
If I have 6022 xx 1023 C atoms I have approximately 12 grams. One step of the Ostwald process for manufacturing nitric acid involves making nitrogen monoxide by oxidizing ammonia in the presence of platinum catalyst which is shown by the equation below. How many molecules per cubic centimeter is this. The mass of argon now added is exactly equal to the neon but argon has a higher gram-atomic weight molar mass than neon. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm.
 Source: convertermaniacs.com
Source: convertermaniacs.com
The change in energy in kJmole of a neutral atom or molecule in the gaseous phase when an electron is added to the atom to form a negative ion. 35453 amu which gives the molar weight of salt as 5844 grams. For other isotopes the isotopic mass usually differs and. One step of the Ostwald process for manufacturing nitric acid involves making nitrogen monoxide by oxidizing ammonia in the presence of platinum catalyst which is shown by the equation below. How many grams of N2 must react to form 17 grams of ammonia.
 Source: youtube.com
Source: youtube.com
For example one mole of sodium chloride NaCl has a molecular mass of 5844 amu Na. Let us use 15 mol for the total moles in the balloon which will be n 2 after the Ar is added. Therefore less than 1 mole of Ar will be added. 26018 g 202 g 20 g 2 g. GRAM MOLECULAR MASS Gram molecular mass is the mass in grams of one mole of a molecular substance.

Critical density grams per cubic centimetre 00696 04819 05356 09092 1103 thermal conductivity watts per metre Kelvin 01513 00491 00177 00094 00057 00036 magnetic susceptibility cgs units per mole 00000019 00000072. One mole of any substance can be defined as the amount of a substance that contains as many particles atoms molecules or ions as there are atoms in 12 g of carbon-12 isotope. This answer will have 3 SF 540 g601 g00185 g0167 g. Let us use 15 mol for the total moles in the balloon which will be n 2 after the Ar is added. For 12 C the atomic mass is exactly 12u since the atomic mass unit is defined from it.
 Source: numerade.com
Source: numerade.com
A kilo-mole is 1000 times as large as a mole. A mole of aluminum is 27g because aluminums relative atomic mass is 27. GRAM MOLECULAR MASS Gram molecular mass is the mass in grams of one mole of a molecular substance. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. If I have 6022 xx 1023 C atoms I have approximately 12 grams.
 Source: pinterest.com
Source: pinterest.com
1591 g 1 mole Cu 2 S 1 mole. If I have 6022 xx 1023 C atoms I have approximately 12 grams. Keep in mind that once one partial pressure is calculated the other can be arrived at by subtraction if so desired. 26018 g 202 g 20 g 2 g. 17 Full PDFs related to this paper.
 Source: slideplayer.com
Source: slideplayer.com
The provided mass of glycine 28 g is a bit more than one-third the molar mass 75 gmol so we would expect the computed result to be a bit greater than one-third of a mole 033 mol. A kilo-mole is 1000 times as large as a mole. GRAM MOLECULAR MASS Gram molecular mass is the mass in grams of one mole of a molecular substance. The same concept is also used to determine the molar quantities of ionic molecules and compounds. Electronegativity of Neon is.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in one mole of neon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






