Your How many grams are in one mole of naoh images are available in this site. How many grams are in one mole of naoh are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams are in one mole of naoh files here. Get all royalty-free photos.
If you’re looking for how many grams are in one mole of naoh pictures information linked to the how many grams are in one mole of naoh topic, you have pay a visit to the right site. Our site frequently provides you with hints for viewing the maximum quality video and image content, please kindly surf and find more enlightening video articles and graphics that match your interests.
How Many Grams Are In One Mole Of Naoh. Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus. Next rinse your buret twice with 10 mL of the NaOH. 5844 x 002 11688 g However there are only 350 ml which is 035 liters. Hence 1 litre would contain.
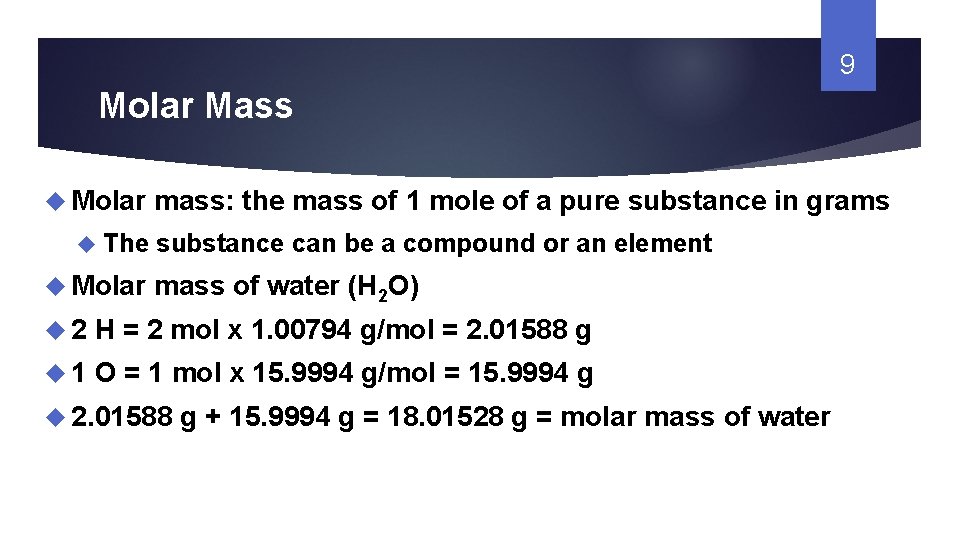 1 Chapter 9 Key Terms Mole Molar Mass From slidetodoc.com
1 Chapter 9 Key Terms Mole Molar Mass From slidetodoc.com
NaOHaq KHC8H4O4aq H2Ol KNaC8H4O4aq Note that one mole of NaOH reacts with one mole of KHP. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. One gram of carbon dioxide contains 136 x 10²¹ number of molecules. How many atoms are in 08288 grams of sodium. A 002 M solution contains 002 moles per liter. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100.
How many molecules are in 1g of co2.
You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus. Next rinse your buret twice with 10 mL of the NaOH. One gram of carbon dioxide contains 136 x 10²¹ number of molecules. How many molecules are in 1g of co2.
 Source: pinterest.com
Source: pinterest.com
You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. 0409 g As stated 1 mole of NaCl weighs 5844 g. NaOHaq KHC8H4O4aq H2Ol KNaC8H4O4aq Note that one mole of NaOH reacts with one mole of KHP. How many atoms are in 08288 grams of sodium. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen.
 Source: slideplayer.com
Source: slideplayer.com
Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus. Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus. 5844 x 002 11688 g However there are only 350 ml which is 035 liters. PROCEDURE STANDARDIZATION OF NAOH Clean your buret and rinse it with deionized water. Next rinse your buret twice with 10 mL of the NaOH.
 Source: youtube.com
Source: youtube.com
Hence 1 litre would contain. PROCEDURE STANDARDIZATION OF NAOH Clean your buret and rinse it with deionized water. 1147 x 1025 atoms. Therefore there would be 035 x 11688 g in total 0409 g For further help this blog post on moles and molarity may be useful. Hence 1 litre would contain.
 Source: slidetodoc.com
Source: slidetodoc.com
Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus. 5844 x 002 11688 g However there are only 350 ml which is 035 liters. 0409 g As stated 1 mole of NaCl weighs 5844 g. A 002 M solution contains 002 moles per liter. 1147 x 1025 atoms.
 Source: slideplayer.com
Source: slideplayer.com
How many grams are in 111 moles of mn3 so4 7. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. One gram of carbon dioxide contains 136 x 10²¹ number of molecules. How many atoms are in 08288 grams of sodium. 0409 g As stated 1 mole of NaCl weighs 5844 g.
 Source: slideplayer.com
Source: slideplayer.com
1147 x 1025 atoms. Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus. Hence 1 litre would contain. How many grams are in 111 moles of mn3 so4 7. Next rinse your buret twice with 10 mL of the NaOH.
 Source: slideplayer.com
Source: slideplayer.com
Therefore there would be 035 x 11688 g in total 0409 g For further help this blog post on moles and molarity may be useful. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. Therefore there would be 035 x 11688 g in total 0409 g For further help this blog post on moles and molarity may be useful. PROCEDURE STANDARDIZATION OF NAOH Clean your buret and rinse it with deionized water. Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus.
 Source: slideplayer.com
Source: slideplayer.com
Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. 1147 x 1025 atoms. Next rinse your buret twice with 10 mL of the NaOH. Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus. How many atoms are in 08288 grams of sodium.
 Source: slideplayer.com
Source: slideplayer.com
Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. PROCEDURE STANDARDIZATION OF NAOH Clean your buret and rinse it with deionized water. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. How many grams are in 111 moles of mn3 so4 7.
 Source: slideplayer.com
Source: slideplayer.com
So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. How many atoms are in 08288 grams of sodium. 1147 x 1025 atoms. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. A 002 M solution contains 002 moles per liter.
 Source: slideplayer.com
Source: slideplayer.com
Therefore there would be 035 x 11688 g in total 0409 g For further help this blog post on moles and molarity may be useful. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. NaOHaq KHC8H4O4aq H2Ol KNaC8H4O4aq Note that one mole of NaOH reacts with one mole of KHP. How many atoms are in 08288 grams of sodium. One gram of carbon dioxide contains 136 x 10²¹ number of molecules.
 Source: pinterest.com
Source: pinterest.com
How many molecules are in 1g of co2. 1147 x 1025 atoms. Hence 1 litre would contain. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. Therefore there would be 035 x 11688 g in total 0409 g For further help this blog post on moles and molarity may be useful.

Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. How many atoms are in 08288 grams of sodium. Next rinse your buret twice with 10 mL of the NaOH. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. PROCEDURE STANDARDIZATION OF NAOH Clean your buret and rinse it with deionized water.
 Source: youtube.com
Source: youtube.com
0409 g As stated 1 mole of NaCl weighs 5844 g. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. One gram of carbon dioxide contains 136 x 10²¹ number of molecules. How many atoms are in 08288 grams of sodium.
 Source: khanacademy.org
Source: khanacademy.org
Hence 1 litre would contain. Also note that the name of KHP is potassium hydrogen phthalate not potassium hydrogen phosphorus. Therefore there would be 035 x 11688 g in total 0409 g For further help this blog post on moles and molarity may be useful. NaOHaq KHC8H4O4aq H2Ol KNaC8H4O4aq Note that one mole of NaOH reacts with one mole of KHP. Next rinse your buret twice with 10 mL of the NaOH.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in one mole of naoh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






