Your How many grams are in one mole of magnesium images are ready. How many grams are in one mole of magnesium are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams are in one mole of magnesium files here. Find and Download all free photos and vectors.
If you’re looking for how many grams are in one mole of magnesium images information linked to the how many grams are in one mole of magnesium interest, you have visit the ideal blog. Our website always gives you suggestions for seeing the highest quality video and picture content, please kindly search and find more enlightening video articles and graphics that fit your interests.
How Many Grams Are In One Mole Of Magnesium. Because the atomic mass of magnesium 24305 amu is slightly more than twice that of a carbon-12 atom 12 amu the mass of 1 mol of magnesium atoms 24305 g is slightly more than. The SI base unit for amount of substance is the mole. Do the same for the sample of oxygen gas. 881 moles of magnesium 243 881 gms.
 Calcualte The Number Of Moles Of Magnesium Present In A Magnesium Ribbon Youtube From youtube.com
Calcualte The Number Of Moles Of Magnesium Present In A Magnesium Ribbon Youtube From youtube.com
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Magnesium Fluoride or 623018064 grams. Type in your own numbers in the form to convert the units. A The mass of 1 mole of carbon is greater than the mass of 1 mole of magnesium. Molar atomic mass of magnesium is 24 g mole-1. 2686g 1 mole MgO 403050g 06664 moles MgO.
The molecular formula for Magnesium is Mg.
Mass 3 moles 24 gmol. Mass 3 moles 24 gmol. Join Login Class 11. Convert grams Magnesium to moles or moles Magnesium to grams Percent composition by element. 124 mole of magnesium x 12 24 x multiplied by 12 divide by 24 x2 atoms. Number of atoms in 5 5 8.
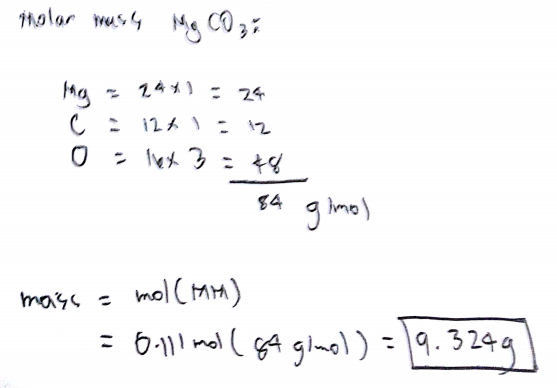 Source: clutchprep.com
Source: clutchprep.com
This problem has been solved. Molar mass of Mg 243050 gmol. Because the atomic mass of magnesium 24305 amu is slightly more than twice that of a carbon-12 atom 12 amu the mass of 1 mol of magnesium atoms 24305 g is slightly more than. 2686g 1 mole MgO 403050g 06664 moles MgO. Join Login Class 11.
 Source: youtube.com
Source: youtube.com
Chlorine Cl Magnesium Mg Molecular weight. So lets find how many moles is 6 grams. Show transcribed image text. Equal number of moles of all the elements contain equal atoms so 112 mole of magnesium also contains x atoms. Suppose you tried to combine 42 grams of magnesium with 45 grams of oxygen.
 Source: youtube.com
Source: youtube.com
Molecular weight of Magnesium Fluoride or grams. How many moles are in 1 gram of magnesium. One mole of magnesium weighs 24 grams. So to convert the mass of magnesium from grams to simply use the elements molar mass as a conversion factor. Show transcribed image text.
 Source: youtube.com
Source: youtube.com
Molecular weight of Magnesium or grams. However one mole of magnesium weighs 2431 gMoles were planned that waySince one mole of MgCl2 consists of one mole of magnesium and two moles of chlorine the mass of one mole of MgCl2 must be the sum of the. From the periodic table we come to know that. Simply find Mg and the atomic mass the biggest number is the same if u have 1. Equal number of moles of all the elements contain equal atoms so 112 mole of magnesium also contains x atoms.
 Source: toppr.com
Source: toppr.com
1 mole of oxygen reacts with 2 moles of magnesium. Q3 How many grams many grams are needed to obtain 15 moles of magnesium oxide. Note that rounding errors may occur so always check the results. B The mass of 1 mole of magnesium is greater than the mass of 1 mole of carbon. Use this page to learn how to convert between moles Magnesium Sulfide and gram.

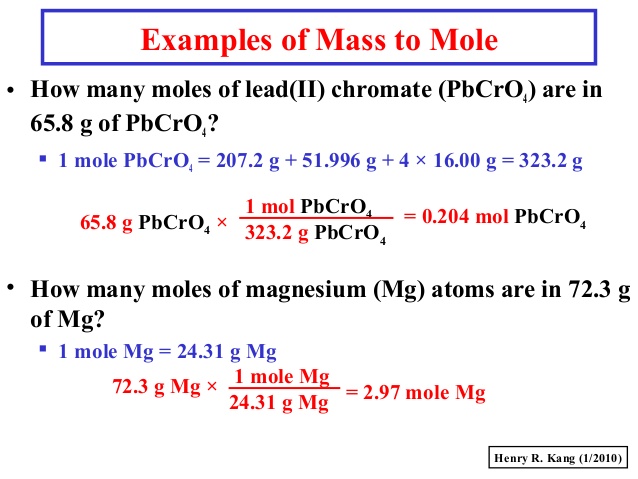
The SI base unit for amount of substance is the mole. Because the atomic mass of magnesium 24305 amu is slightly more than twice that of a carbon-12 atom 12 amu the mass of 1 mol of magnesium atoms 24305 g is slightly more than. 1 Mole of magnesium 24305 grams The 24305 comes from the periodic table of elements. Join Login Class 11. 1067g 1 mole O2 320g 03334 moles O2.
 Source: renewdatabase.weebly.com
Source: renewdatabase.weebly.com
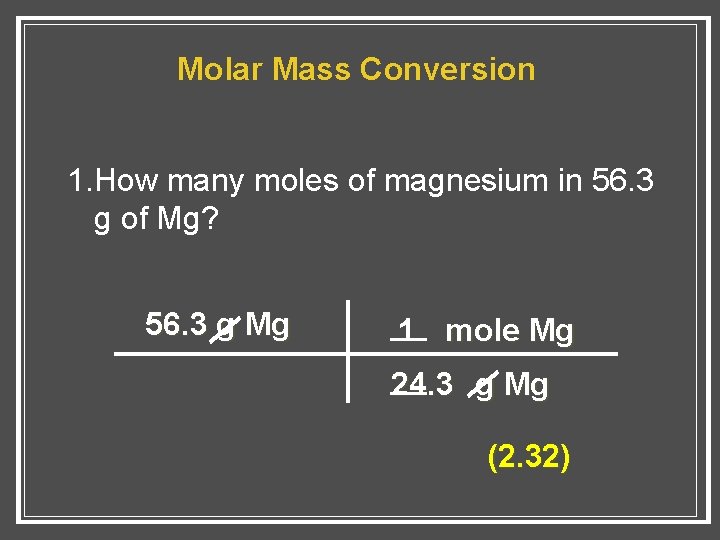
15 moles of oxygen will react with. Type in your own numbers in the form to convert the units. 1 grams Magnesium is equal to 0041143797572516 mole. Each atom has a different size and therefore a different mass. 1 mole of magnesium 243 gm.

Q3 How many grams many grams are needed to obtain 15 moles of magnesium oxide. CAS Registry Number CAS RN. Note that rounding errors may occur so always check the results. 1 mole of oxygen reacts with 2 moles of magnesium. The knowledge of atomic mass of magnesium is also required to solve the problem.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles Magnesium or 24305 grams. Then to calculate moles of magnesium. Molecular weight of Magnesium Fluoride or grams. Mass 72 g. Note that rounding errors may occur so always check the results.
 Source: topperlearning.com
Source: topperlearning.com
Type in your own numbers in the form to convert the units. Weight to volume volume to weight price mole to volume and weight mass and molar concentration density. The relative masses of each element can be found on the periodic table. Therefore 24 gram 1 mole. Simply find Mg and the atomic mass the biggest number is the same if u have 1.
 Source: slideplayer.com
Source: slideplayer.com
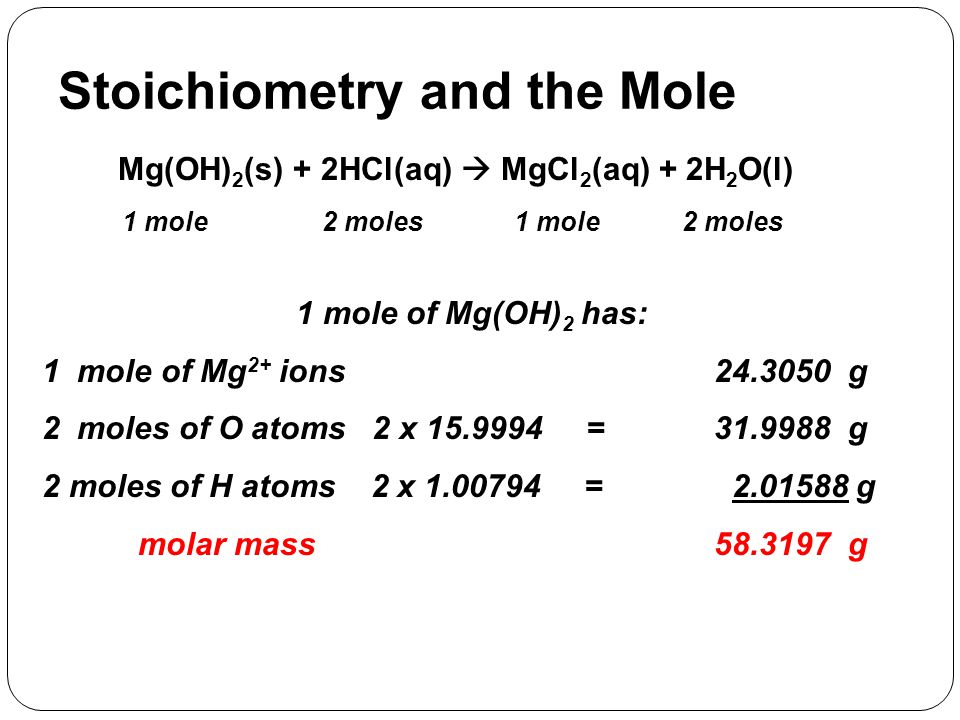
See the answer See the answer See the answer done loading. Moles mass gmolar mass gmol Mass moles molar mass. The figure means that the mass of one mole of Mg is 243g. Note that rounding errors may occur so always check the results. See the answer See the answer See the answer done loading.

Ive concluded the answer is 002 moles of H2 but Im doubting myself a lot because I dont understand the aspects of the. 1 mole is equal to 1 moles Magnesium or 24305 grams. 1 mole is equal to 1 moles Magnesium Sulfide or 5637 grams. Suppose you tried to combine 42 grams of magnesium with 45 grams of oxygen. Number of atoms in 5 5 8.
 Source: brainly.com
Source: brainly.com
Hence the number of moles present in 425 grams of magnesium oxide are 10625moles. For example one atom of magnesium weighs 2431 amu atomic mass units. Finally do the same for the magnesium oxide. Use this page to learn how to convert between grams Magnesium and mole. So lets find how many moles is 6 grams.

Hence the number of moles present in 425 grams of magnesium oxide are 10625moles. 1 mole is equal to 1 moles Magnesium or 24305 grams. 1 mole of oxygen reacts with 2 moles of magnesium. 5 gram Fe is. 2nd to calculate the grams of magnesium needed.
 Source: slidetodoc.com
Source: slidetodoc.com
1 mole of oxygen reacts with 2 moles of magnesium. Do the same for the sample of oxygen gas. 15 2 3 moles. 2nd to calculate the grams of magnesium needed. D There are more atoms in 1 mole of magnesium than in 1 mole of carbon.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams are in one mole of magnesium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






