Your How many grams are in one mole of cu images are ready in this website. How many grams are in one mole of cu are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams are in one mole of cu files here. Get all royalty-free vectors.
If you’re looking for how many grams are in one mole of cu pictures information connected with to the how many grams are in one mole of cu interest, you have come to the right blog. Our website frequently provides you with suggestions for seeing the highest quality video and image content, please kindly search and find more enlightening video articles and images that fit your interests.
How Many Grams Are In One Mole Of Cu. Your goal is to determine the mass in grams of two moles of carbon. Therefore 1 mole Cu 63546 g Cu. We assume you are converting between moles CuSO4 5H2O and gram. Use the molar masses of copper and of silver nitrate to determine how many moles of each you have.
 What Is The Average Mass Of One Atom Of Copper Youtube From youtube.com
What Is The Average Mass Of One Atom Of Copper Youtube From youtube.com
How many moles of copper would be needed to make 1 mole of Cu O. How many grams of copper would you need. Molecular weight of CuOH2 or grams This compound is. From the above equation Mol ratio of Cu. Use the molar mass of copper to calculate how many moles you have in your sample. AgNO3 1.
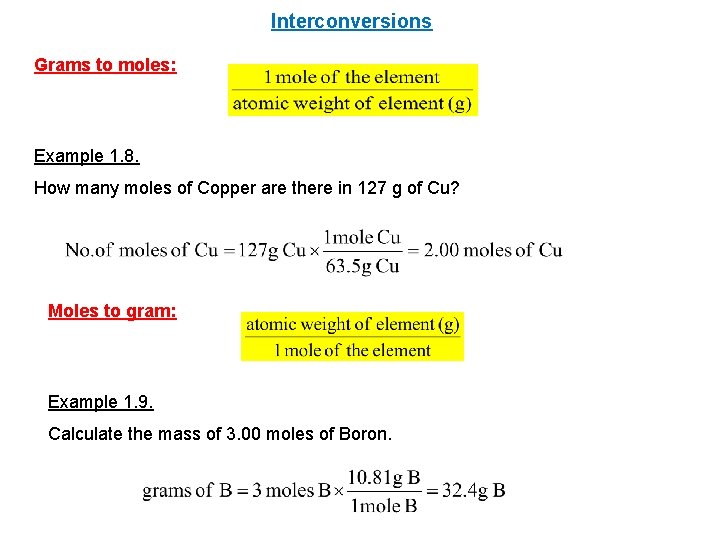
Your goal is to determine the mass in grams of two moles of carbon.
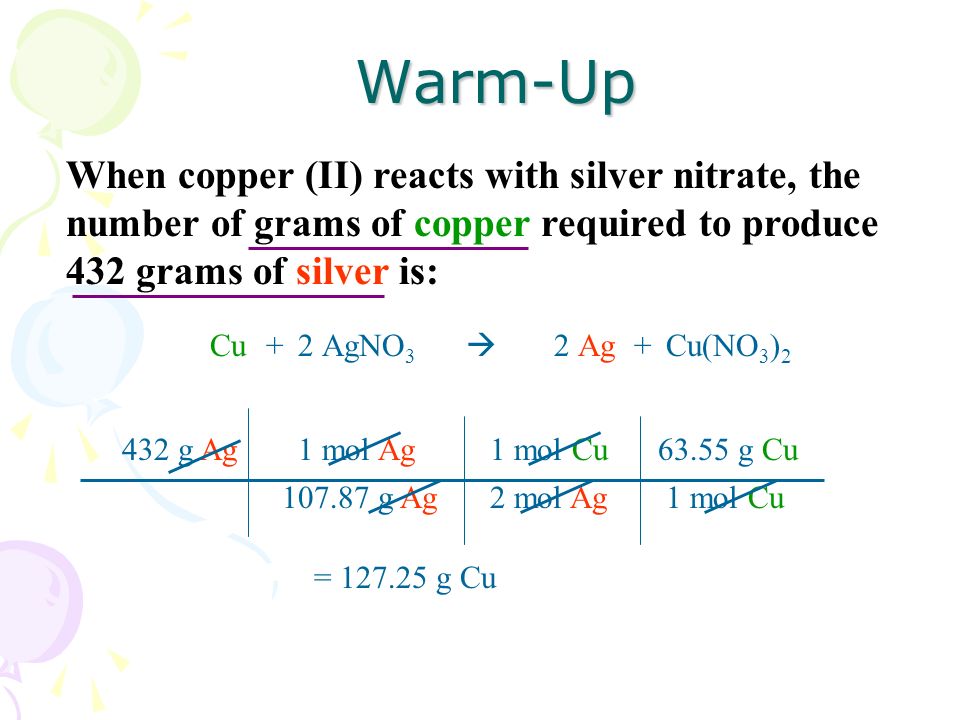
1 mole is equal to 1 moles CuSO4 5H2O or 249685 grams. Use this page to learn how to convert between moles Copper and gram. Molar mass mass of one mole of a substance. 001574moles Cu 2amoles Ag 1mole Cu 003148 moles Ag. NCu mM 350g63546gmol 005508 mol Cu. 3910 grams is the molar mass of one mole of K.
 Source: slidetodoc.com
Source: slidetodoc.com
G 15L 010M II. 3910 grams is the molar mass of one mole of K. From the above equation Mol ratio of Cu. The answer is 0010250031057594. How many grams of copper would you need.
 Source: pinterest.com
Source: pinterest.com
Since 6022 1023atoms mol is a fraction divide by multiplying by its reciprocal. How many grams of copper would you need. Use dimensional analysis to calculate the mass of 4 atoms of Cu. Convert grams Cu to moles or moles Cu to grams Percent composition by element. You can view more details on each measurement unit.
 Source: nl.pinterest.com
Source: nl.pinterest.com
The number of atoms can also be calculated using Avogadros Constant 60221417910 23 one mole of substance. Cu molecular weight. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS. 148 1025 atoms Cu 1mol Cu 6022 1023 atoms Cu 246 mol Cu rounded to three significant figures There are. For NaCl the molar mass is 5844 gmol.
 Source: slidetodoc.com
Source: slidetodoc.com
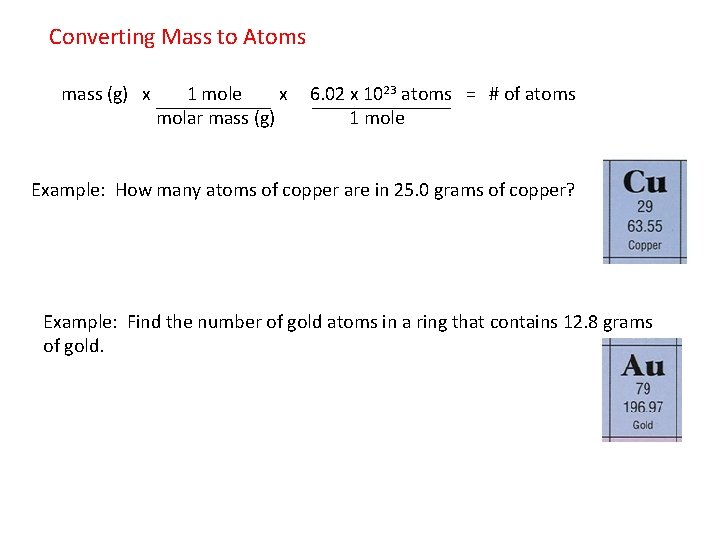
How many moles of barium are in a sample containing 425 x 1026 atoms of barium. Use this page to learn how to convert between moles Cu and gram. How many grams of copper would you need. 895 g Ag x 1 mol Ag x 1 mol Cu x 6355 g Cu 264 g Cu. 425 x 1026 atoms of barium 1 mol 706 mol 602 x 1023 atoms 3.
 Source: slidetodoc.com
Source: slidetodoc.com
It is equal to Avogadros number NA namely 6022 x10 23If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. In addition to showing the ratio of atoms in a molecule what else do the subscripts in a The subscripts show us what is in a molecule. Use the molar mass of copper to calculate how many moles you have in your sample. 15 L 10 mol AgNO3 1 L 48 g Cu Cu 2AgNO3 2Ag CuNO32 1 mol Cu 2 mol AgNO3. Convert grams Cu to moles or moles Cu to grams Percent composition by element.
 Source: youtube.com
Source: youtube.com
We assume you are converting between moles CuSO4 5H2O and gram. Often means the mass in grams of one mole not one molecule of a compound eg 18 gmol for H 2 O 32 gmol for O 2. How many moles CuOH2 in 1 grams. Molecular weight of CuSO4 5H2O or grams The SI base unit for amount of substance is the mole. Molar mass of Cu 63546 gmol.
 Source: pinterest.com
Source: pinterest.com
Molecular weight of CuOH2 or grams This compound is. Your goal is to determine the mass in grams of two moles of carbon. The mole ratio tells you that for every 1 mole of Cu that reacts 2 moles of Ag are produced. Use the molar mass of copper to calculate how many moles you have in your sample. That much copper would have needed.
 Source: slideplayer.com
Source: slideplayer.com
G 15L 010M II. Since 6022 1023atoms mol is a fraction divide by multiplying by its reciprocal. Molten iron and carbon monoxide are produced in a blast furnace by the reaction of ironIII oxide and coke pure carbon. Molecular weight of CuOH2 or grams This compound is. Often means the mass in grams of one mole not one molecule not one formula unit of a compound.
 Source: slideplayer.com
Source: slideplayer.com
Grams can be canceled leaving the moles of K. NAg 005508 mol Cu2molAg1molCu 01102 mol Ag. So 355 mol of AgNO3 requires ½ x 355 mol of Cu 1775 mol of Cu. Divide the given number of atoms by 6022 1023atoms mol. AgNO3 1.
 Source: slidetodoc.com
Source: slidetodoc.com
NCu mM 350g63546gmol 005508 mol Cu. How many grams is in 1000 moles of calcium Ca. 1 mole is equal to 1 moles CuSO4 5H2O or 249685 grams. NCu mM 350g63546gmol 005508 mol Cu. Since 6022 1023atoms mol is a fraction divide by multiplying by its reciprocal.
 Source: socratic.org
Source: socratic.org
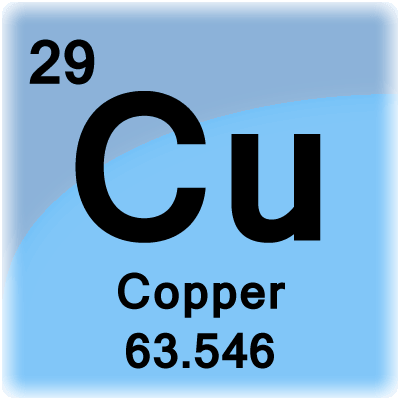
1 mole is equal to 1 moles Cu or 63546 grams. How many grams is in 1000 moles of calcium Ca. Molecular weight of Copper or grams. 1271g Grams of oxygen. For NaCl the molar mass is 5844 gmol.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles Copper or 63546 grams. Cu molecular weight. Note that rounding errors may occur so always check the results. Use the molar masses of copper and of silver nitrate to determine how many moles of each you have. Using mole ratio we find moles of Ag.
 Source: slidetodoc.com
Source: slidetodoc.com
How many moles of copper would be needed to make 1 mole of Cu O. We assume you are converting between moles CuSO4 5H2O and gram. Molar mass of Cu 63546 gmol. 1359g 1 mole AgN O3 16987g 8000 moles AgN O3. How many moles of copper would be needed to make 1 mole of Cu 2 O.
 Source: youtube.com
Source: youtube.com
100g 1 mole Cu 63546 g 001574 moles Cu. Each jar holds exactly one mole of carbon. Notice that you have insufficient silver nitrate to allow for all of the moles of copper to react. 1 mole is equal to 1 moles Cu or 63546 grams. 001574moles Cu 2amoles Ag 1mole Cu 003148 moles Ag.
 Source: slidetodoc.com
Source: slidetodoc.com
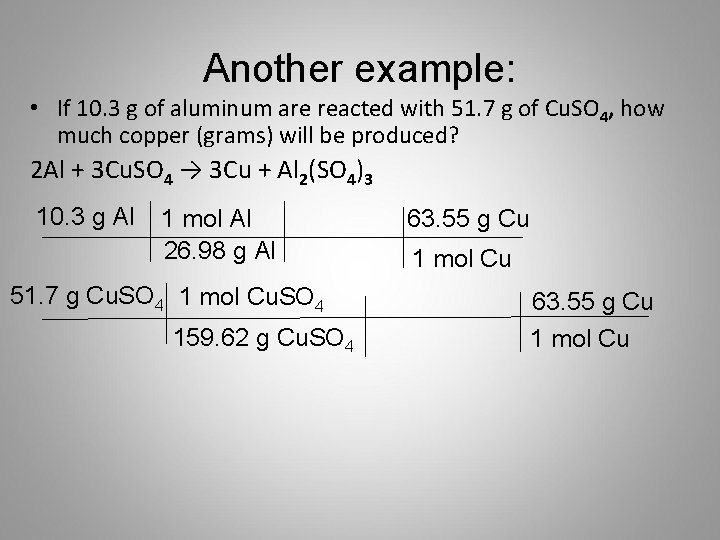
15 L 10 mol AgNO3 1 L 48 g Cu Cu 2AgNO3 2Ag CuNO32 1 mol Cu 2 mol AgNO3. Often means the mass in grams of one mole not one molecule of a compound eg 18 gmol for H 2 O 32 gmol for O 2. The mole ratio tells you that for every 1 mole of Cu that reacts 2 moles of Ag are produced. 381g 1 mole Cu 6355g 600 moles Cu. G 6355 g Cu 1 mol Cu Stoichiometry Problems How many grams of Cu are required to react with 15 L of 010M AgNO3.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in one mole of cu by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






