Your How many grams are in one mole of ch4 images are ready. How many grams are in one mole of ch4 are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams are in one mole of ch4 files here. Download all royalty-free vectors.
If you’re searching for how many grams are in one mole of ch4 pictures information linked to the how many grams are in one mole of ch4 keyword, you have come to the right site. Our website always gives you suggestions for viewing the maximum quality video and image content, please kindly search and locate more informative video content and images that match your interests.
How Many Grams Are In One Mole Of Ch4. You can view more details on each measurement unit. If one mole of carbon monoxide has a mass of 2801 g and one mole of carbon dioxide has a mass of 4401 g it follows that the reaction produces 4401 g of carbon dioxide for every 2801 g. Molar mass CH4 160 gmol G. How many grams of O 2 must react with excess.
 How To Convert Moles Of Ch4 To Grams Youtube From youtube.com
How To Convert Moles Of Ch4 To Grams Youtube From youtube.com
8 g CH4 12 mole CH4. Type in your own numbers in the form to convert the units. You can view more details on each measurement unit. The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results. Avogadros number is equal to 60221023 moleculesmole.
From the following balanced equation CH4g2O2g CO2g2H2Og how many grams of H2O can be formed when 125g CH4 are combined with 1251023 molecules O2.
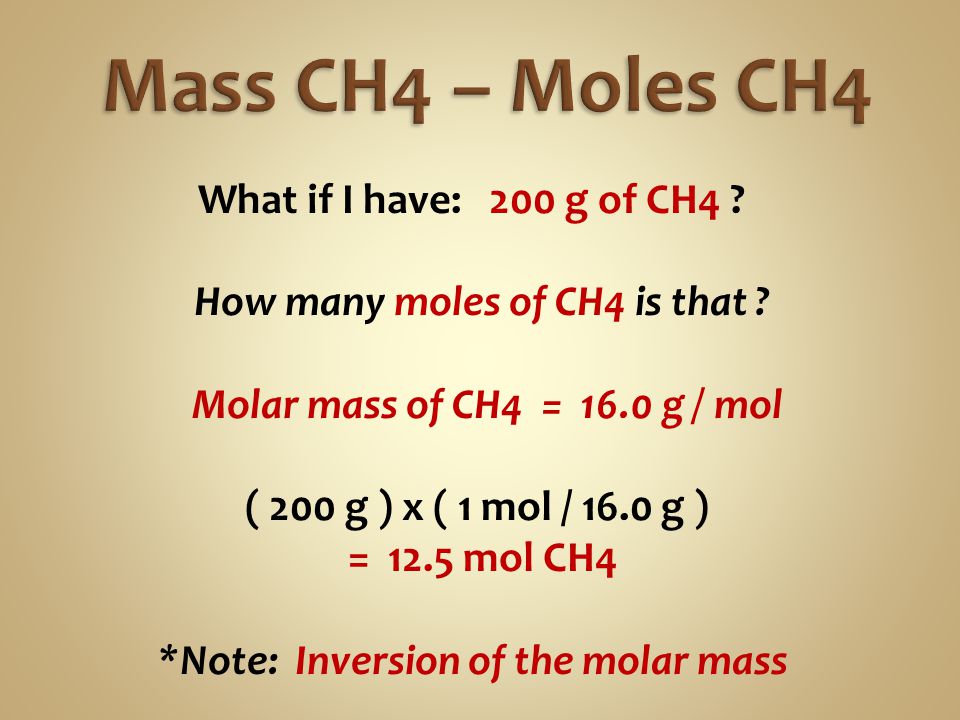
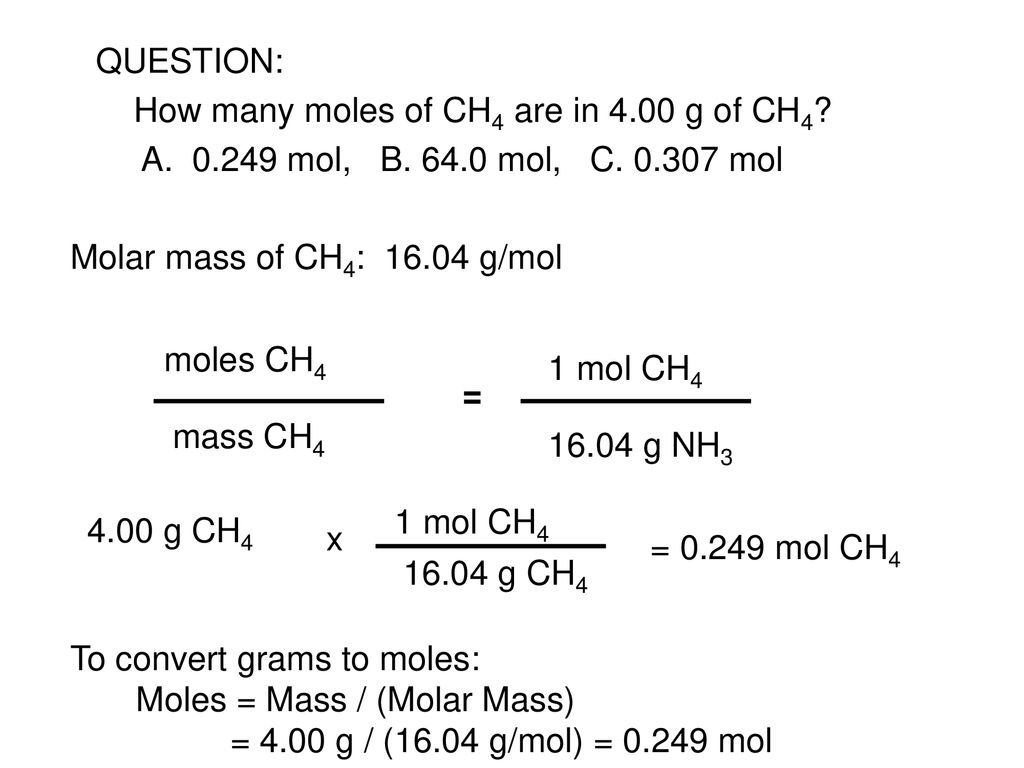
What is the mass of 1 mol of CH4. A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. The technique used can be applied to any mole to gram conversion. Type in your own numbers in the form to convert the units. The mole ratio of CH4 to H2O is 1 2. The answer is 1604246.
 Source: slideplayer.com
Source: slideplayer.com
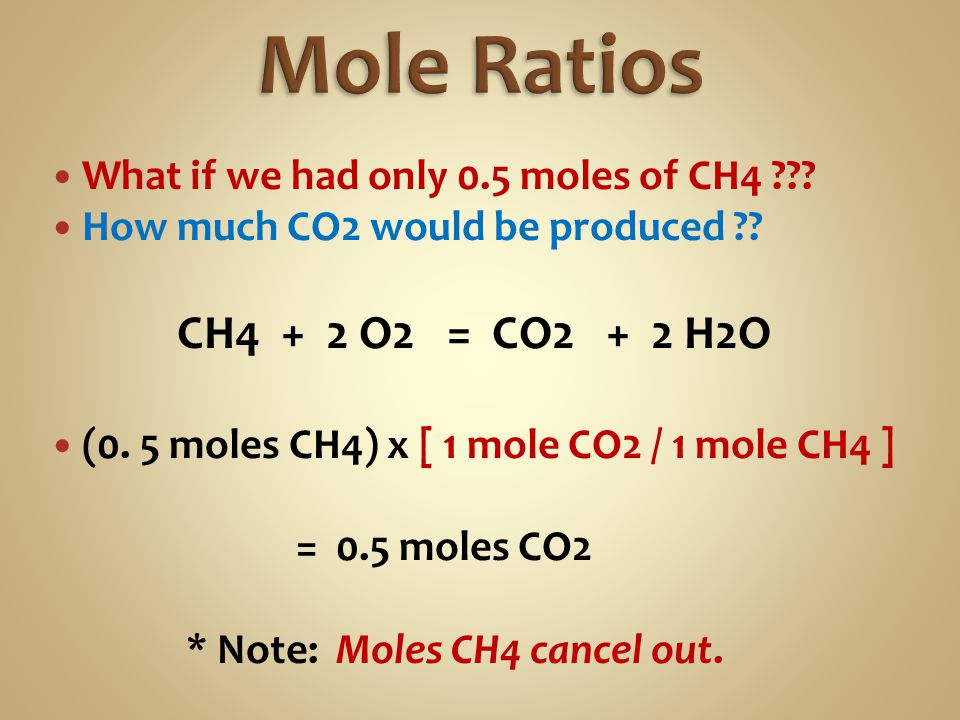
Now each molecule of CH4 is made up of one atom of carbon and 4 atoms of hydrogen. Now 3 moles would react with 12 3 moles of CH4. How many moles are in CH4. How many moles of O 2 are required to react with 172 moles of CH 4. Therefore there are 301 times 1023 atoms in 16g of methane.
 Source: brainly.in
Source: brainly.in
If you WANTED 2 moles ofCH4 you need to multiply this molecular weight by 2 moles to get32 grams the moles cancel. Therefore the molar mass of methane is 16g. The answer is 1604246. G N 2 947 g H 2 x 1 mol H 2 x 1 mol N 2 x 2801g N 2 2016 g H 2 3 mol H 2 1 molN2 439 g N 2 Stoichiometry CH 4 2 O 2 CO 2 2 H 2O Answer the following questions. If one mole of carbon monoxide has a mass of 2801 g and one mole of carbon dioxide has a mass of 4401 g it follows that the reaction produces 4401 g of carbon dioxide for every 2801 g.
 Source: meritnation.com
Source: meritnation.com
1 grams CH4 is equal to 0062334579609362 mole. Use 60221023 mol1 for Avogadros number. How many moles of CO2 are produced from the combustion of 100 g of. How many moles of CO2 will be produced from 200 g CH4 assuming o2 is available in excess. There are 18021 1024 molecules of CH_4 in 48 grams of CH_4.

Hence number of moles of CO₂ 125. Grams to teaspoons for sugar granulated Grams to teaspoons Grams to teaspoons 1 gram 024 tsp 20 grams 48 tsp 2 grams 0478 tsp 30 grams 717 tsp 3 grams 0717 tsp 40 grams 956 tsp 4 grams 0956 tsp 50 grams 11. We know that the molar mass of CH4 is 16 g. Hence number of moles of CO₂ 125. Now each molecule of CH4 is made up of one atom of carbon and 4 atoms of hydrogen.
 Source: toppr.com
Source: toppr.com
For example the atomic mass of methane CH4is 12 amu for the carbon plus 4 x 1 amu for the four hydrogens for a total of 16 amu. We say that one mole of methane has a mass of. Use 60221023 mol1 for Avogadros number. 1mole CH4 16 gram. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
How many atoms of hydrogen are present in 16 grams of methane. If one mole of carbon monoxide has a mass of 2801 g and one mole of carbon dioxide has a mass of 4401 g it follows that the reaction produces 4401 g of carbon dioxide for every 2801 g. Hence number of moles of CO₂ 125. Molecular weight of CH4 or mol This compound is also known as Methane. Molar mass of CH416g.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. Avogadros number is equal to 60221023 moleculesmole. The SI base unit for amount of substance is the mole. 1 grams CH4 is equal to 0062334579609362 mole. Molar mass CH4 16gmol.
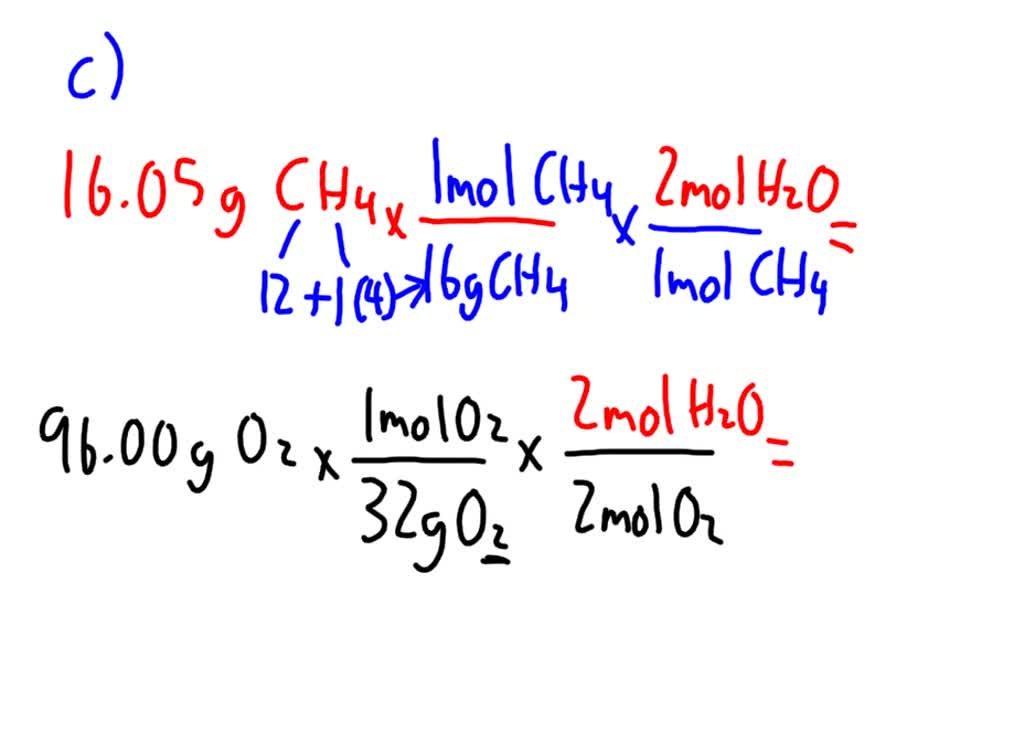 Source: numerade.com
Source: numerade.com
If you mean CH4 1 Carbon. From the following balanced equation CH4g2O2g CO2g2H2Og how many grams of H2O can be formed when 125g CH4 are combined with 1251023 molecules O2. We say that one mole of methane has a mass of. Or molar mass of an element is equal to the molecular weight. How many moles of CO2 are produced from the combustion of 100 g of.
 Source: toppr.com
Source: toppr.com
It is equal to Avogadros number NA namely 6022 x10 23If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. Type in your own numbers in the form to convert the units. Therefore the molar mass of methane is 16g. The mole ratio of CH4 to H2O is 1 2. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
Now 3 moles would react with 12 3 moles of CH4. How many moles of CO2 are produced from the combustion of 100 g of. Methane CH4 C 12 grams per mole H 1 gram per mole so CH4 16 gramsmole of which 4 are H so 4 grams. From the following balanced equation CH4g2O2g CO2g2H2Og how many grams of H2O can be formed when 125g CH4 are combined with 1251023 molecules O2. Therefore there are 301 times 1023 atoms in 16g of methane.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. We say that one mole of methane has a mass of. How many grams of H 2O will form when 109 moles of CH 4 react with excess O 2. We know that the molar mass of CH4 is 16 g. Molar mass CH4 160 gmol G.
 Source: youtube.com
Source: youtube.com
A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. G N 2 947 g H 2 x 1 mol H 2 x 1 mol N 2 x 2801g N 2 2016 g H 2 3 mol H 2 1 molN2 439 g N 2 Stoichiometry CH 4 2 O 2 CO 2 2 H 2O Answer the following questions. How many moles of O 2 are required to react with 172 moles of CH 4. The SI base unit for amount of substance is the mole. Now each molecule of CH4 is made up of one atom of carbon and 4 atoms of hydrogen.
 Source: youtube.com
Source: youtube.com
1mole CH4 16 gram. Therefore the molar mass of methane is 16g. We assume you are converting between grams CH4 and mole. 1 grams CH4 is equal to 0062334579609362 mole. CH4 2O2 CO2 2H2O.
 Source: toppr.com
Source: toppr.com
Note that rounding errors may occur so always check the results. How many atoms of hydrogen are present in 16 grams of methane. From the following balanced equation CH4g2O2g CO2g2H2Og how many grams of H2O can be formed when 125g CH4 are combined with 1251023 molecules O2. 4 rows The answer is 1604246. There are 18021 1024 molecules of CH_4 in 48 grams of CH_4.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles CH4 or 1604246 grams. How many spoons are 40 grams. So number of covalent bonds in 8 g CH4 3011 1023 4 1204 1024. Number of moles of methane wMm 2016 125.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams are in one mole of ch4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






