Your How many grams are in one mole of aluminum chloride images are available in this site. How many grams are in one mole of aluminum chloride are a topic that is being searched for and liked by netizens today. You can Get the How many grams are in one mole of aluminum chloride files here. Find and Download all royalty-free photos.
If you’re looking for how many grams are in one mole of aluminum chloride pictures information connected with to the how many grams are in one mole of aluminum chloride keyword, you have come to the right site. Our website always provides you with hints for viewing the maximum quality video and picture content, please kindly search and locate more enlightening video content and graphics that match your interests.
How Many Grams Are In One Mole Of Aluminum Chloride. 279 moles of aluminum c. The molecular weight of AlCl3 is 13334 gmole. Each mole of the salt will contain one mole of Calcium. The number of moles in 0255 g is.
 Mole Calculations Moles To Mass N N N From slidetodoc.com
Mole Calculations Moles To Mass N N N From slidetodoc.com
Molecular weight of aluminum chloride or grams. Each mole of the salt will contain one mole of Calcium and two moles of Chlorine. Furthermore how many moles of chloride ions are there in 2 moles of calcium chloride. How many grams of aluminum oxide are formed when 250 grams of Aluminum are reacted with oxygen gas. Consequently how many moles of chloride ions are there in 2 moles of calcium chloride. 1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams.
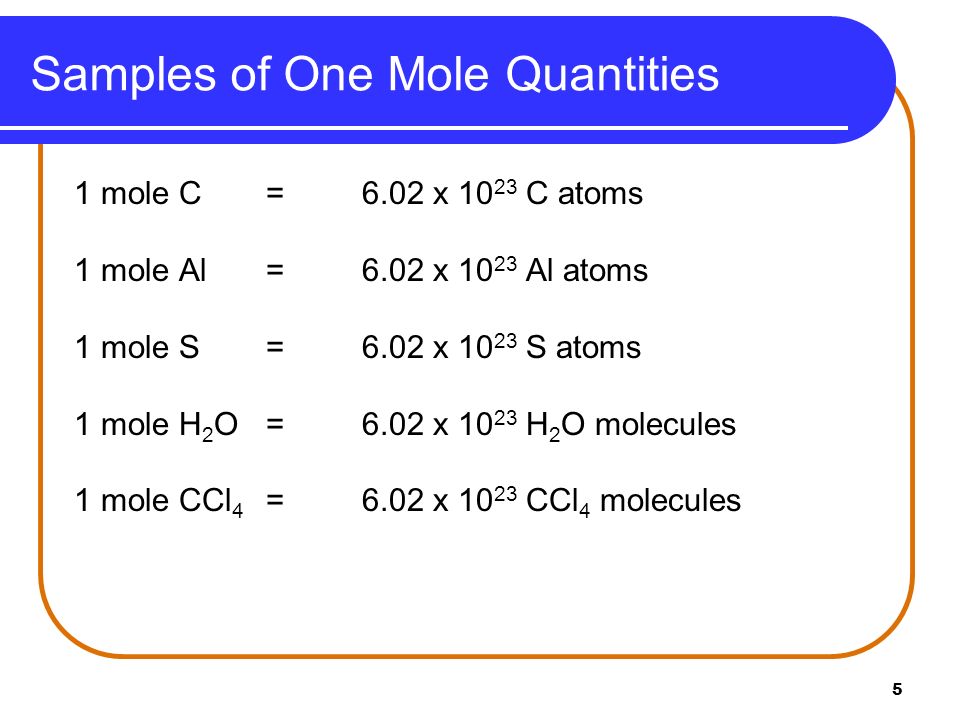
How many moles are in AlCl3.
Molar mass of AlCl3 13334 gmol moles of AlCl3 13413334 01005 moles. Each mole of the salt will contain one mole of Calcium and two moles of Chlorine. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles aluminum chloride or 133340538 grams. 1 grams AlCl3 is equal to 00074995947593972 mole. 833 moles CO2 X.
 Source: pinterest.com
Source: pinterest.com
Each mole of the salt will contain one mole of Calcium and two moles of Chlorine. 2 I 2 X 12690 25380 g I 2 mol EXAMPLE. 837 moles of chloride d. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2. Aluminium Chloride molecular weight.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass of AlCl3 13334 gmol moles of AlCl3 13413334 01005 moles. 1 mol FeOH 3 1068 g FeOH 3 00616 mol FeCl 3 x x 658 g FeOH 3 1 mol FeCl 3 1 mol FeOH 3 In the foregoing EXAMPLES we have twice calculated that 658 g FeOH 3 can be obtained from 100 g FeCl 3 by chemical reaction 1. How many kg of Titanium III chloride was produced from 52 kg of Titanium IV chloride. The molecular formula for Calcium Chloride is CaCl2 ie. Amount of AlOH3 produced 0100578 784 g.
 Source: slideplayer.com
Source: slideplayer.com
837 moles of chloride d. How many moles of barium sulfate are produced from 01 mole of barium chloride. Answer 1 of 5. Subsequently theres 74996 mols of Al. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2.
 Source: slideplayer.com
Source: slideplayer.com
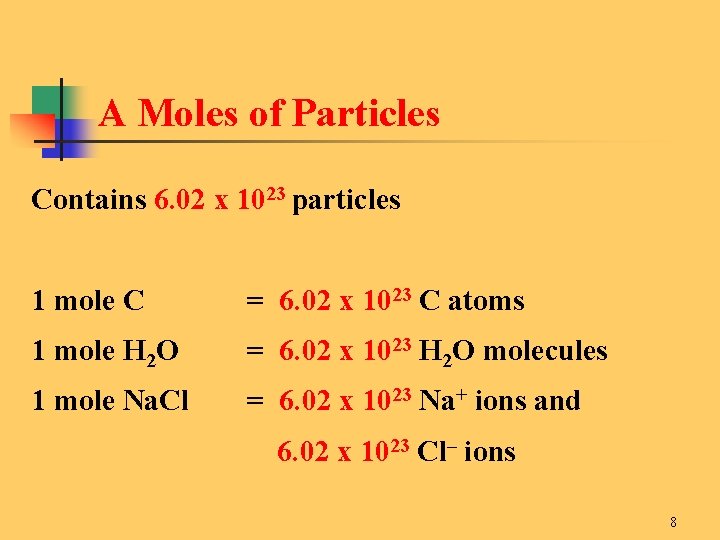
How many moles of barium sulfate are produced from 01 mole of barium chloride. 7500 mols of Al. The molecular formula for Calcium Chloride is CaCl2 ie. One mole of sodium chloride contains 602 x 10 23 molecules of sodium chloride etc. The SI base unit for amount of substance is the mole.
 Source: slidetodoc.com
Source: slidetodoc.com
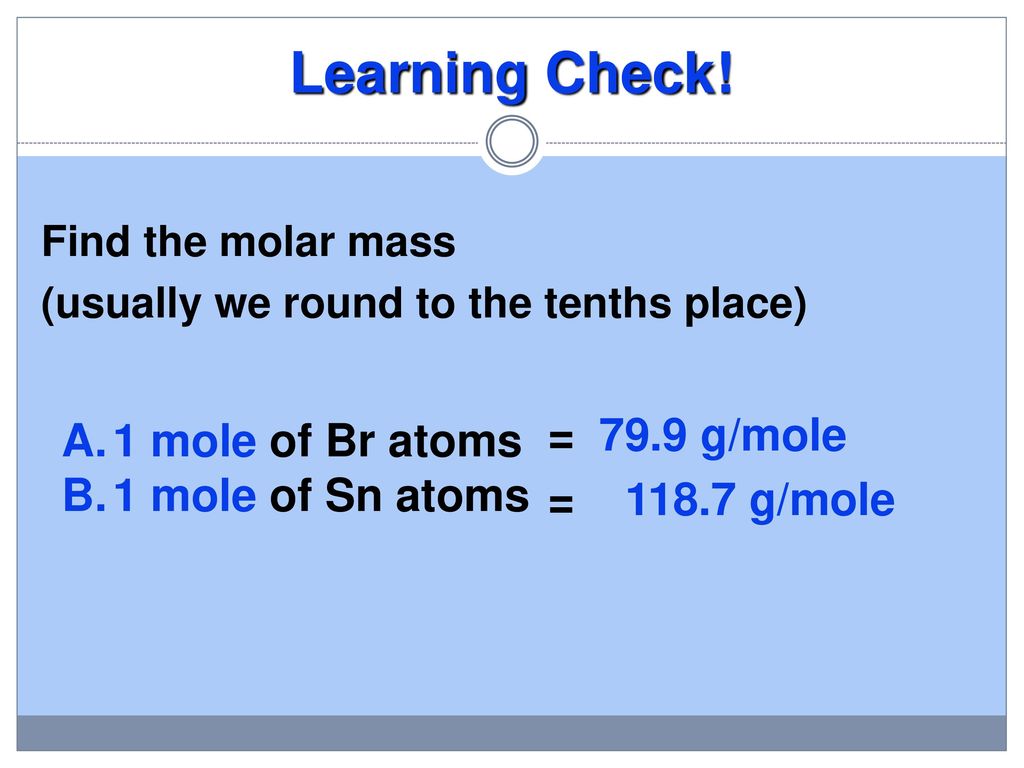
Amount of AlOH3 produced 0100578 784 g. Molar mass of NaOH 40 gmol. How many moles of oxygen gas are required to make 833 moles of carbon dioxide. 000191 moles AlCl3 X 3 chloridesmole of AlCl3. What is mole of aluminum chloride.
 Source: slideplayer.com
Source: slideplayer.com
1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams. Calculate the molar mass gram formula weight of a mole of aluminum sulfate Al 2 SO 4 3. 15232422 pounds lbs of Aluminum chloride fit into 1 cubic foot. Molar mass of AlCl3 13334 gmol moles of AlCl3 13413334 01005 moles. Barium chloride and sodium sulfate react according to the following equation.
 Source: slidetodoc.com
Source: slidetodoc.com
How many grams are in 300 moles of aluminum. Consequently how many moles of chloride ions are there in 2 moles of calcium chloride. How many moles of barium sulfate are produced from 01 mole of barium chloride. 15232422 pounds lbs of Aluminum chloride fit into 1 cubic foot. The molecular formula for aluminum chloride is AlCl3.
 Source: slidetodoc.com
Source: slidetodoc.com
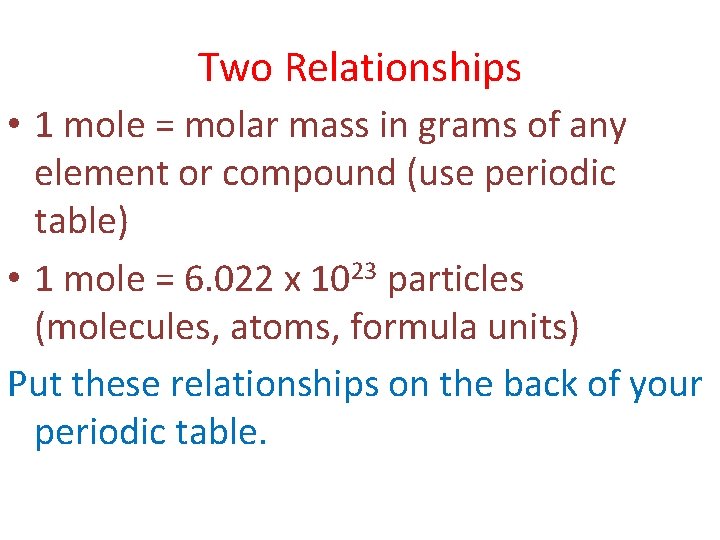
1 mole is the same as 1 moles Aluminium Chloride or 133340538 grams. The molecular formula for aluminum chloride is AlCl3. 000191 moles AlCl3 X 3 chloridesmole of AlCl3. The SI base unit for quantity of substance is the mole. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2.
 Source: slidetodoc.com
Source: slidetodoc.com
Aluminum chloride weighs 244 gram per cubic centimeter or 2 440 kilogram per cubic meter ie. The molecular formula for Aluminium Chloride is AlCl3. Answer 1 of 5. How many grams are in 300 moles of aluminum. One may also ask how many moles of chloride ions are there in 2 moles of calcium chloride.
 Source: studylib.net
Source: studylib.net
How many kg of Titanium III chloride was produced from 52 kg of Titanium IV chloride. 279 moles of aluminum chloride b. The SI base unit for amount of substance is the mole. Amount of AlOH3 produced 0100578 784 g. 1 mole is equal to 1 moles MgCl2 or 95211 grams.
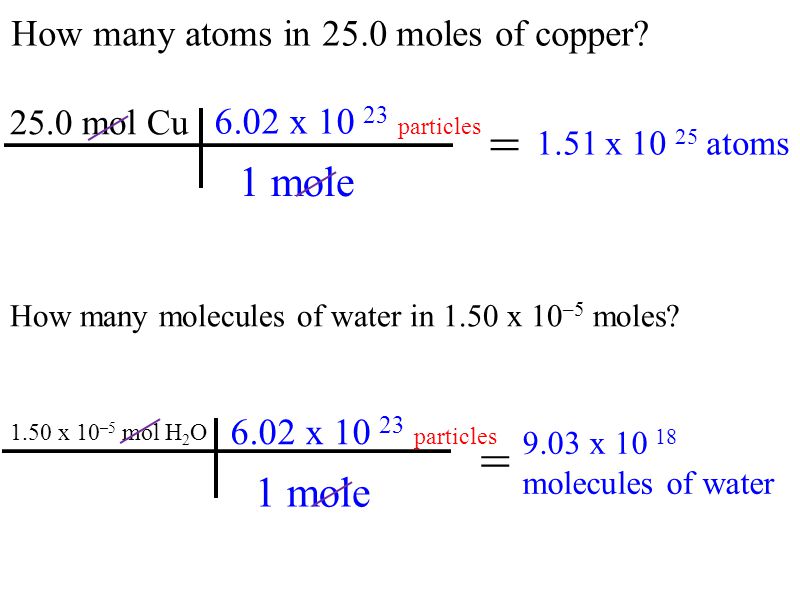 Source: slideplayer.com
Source: slideplayer.com
The molecular weight of AlCl3 is 13334 gmole. How many grams are in 300 moles of aluminum. Based on the reaction stoichiometry. One mole of sodium chloride contains 602 x 10 23 molecules of sodium chloride etc. How many moles of barium sulfate are produced from 01 mole of barium chloride.
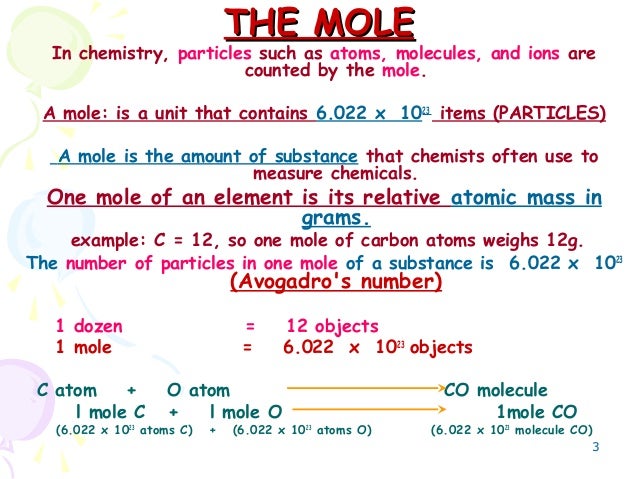 Source: slideshare.net
Source: slideshare.net
The molecular formula for Calcium Chloride is CaCl2 ie. Each mole of the salt will contain one mole of Calcium and two moles of Chlorine. This compound is also known as Magnesium Chloride. 15232422 pounds lbs of Aluminum chloride fit into 1 cubic foot. A 02000 grams sample of aluminum metal is added to a solution containing 10000grams of an unknown compound made of platinum and chloride ion.
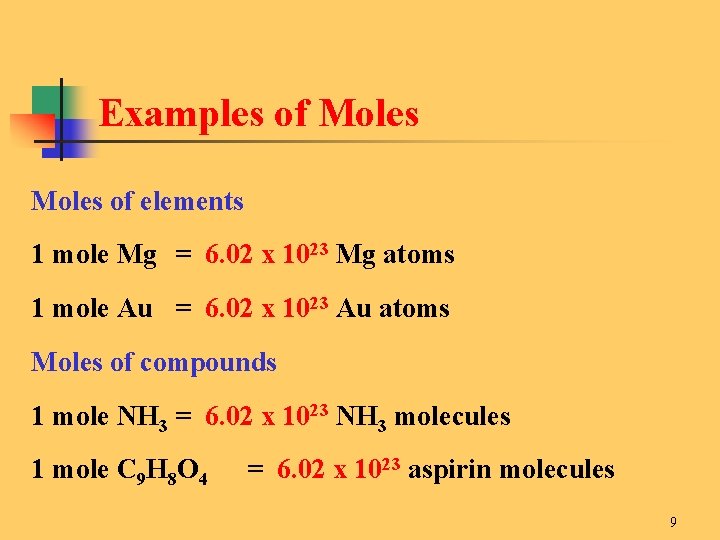 Source: slidetodoc.com
Source: slidetodoc.com
At 25C 77F or 29815K at standard atmospheric pressure. What number of grams are in a single mole of aluminum chloride. 7500 mols of Al. 279 moles of aluminum c. The number of moles in 0255 g is.
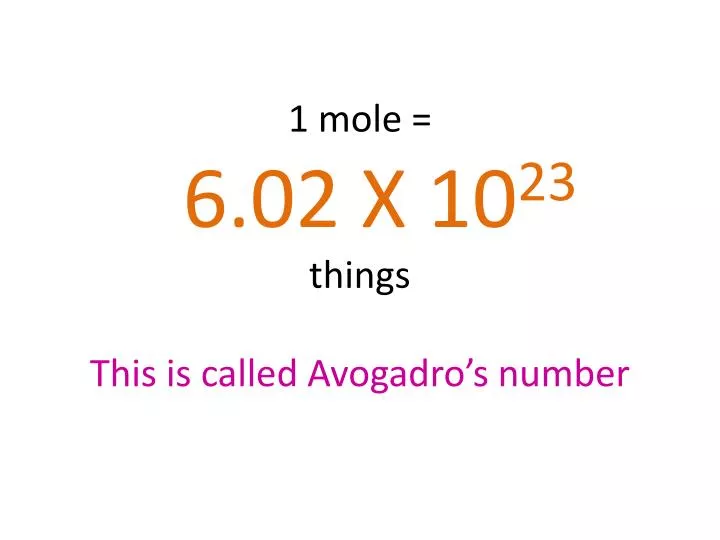 Source: slideserve.com
Source: slideserve.com
837 moles of chloride d. 52 Kg TiCl4 X. How many grams of potassium chloride is required for a 225mL sample with concentration of 150 molL. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2. Round to 2 decimal places.
 Source: slideplayer.com
Source: slideplayer.com
Aluminum chloride is an ionic salt which means it is the combination of a metal ion aluminum and a non-metal ion chloride. How many grams of potassium chloride is required for a 225mL sample with concentration of 150 molL. Amount of AlOH3 produced 0100578 784 g. Answer 1 of 5. 1 mole is equal to 1 moles aluminum chloride or 133340538 grams.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams are in one mole of aluminum chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






