Your How many grams are in one mole images are available. How many grams are in one mole are a topic that is being searched for and liked by netizens now. You can Get the How many grams are in one mole files here. Download all royalty-free vectors.
If you’re searching for how many grams are in one mole pictures information linked to the how many grams are in one mole topic, you have pay a visit to the right blog. Our website frequently gives you hints for downloading the maximum quality video and image content, please kindly hunt and find more informative video content and graphics that fit your interests.
How Many Grams Are In One Mole. One mole of N2 is 28 grams Nitrogen has an atomic weight of 14 and one mole of N2 would be 214 28g How many grams are in 1 mole of KCl. Use this page to learn how to convert between moles Si and gram. The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results.
 Molality Easy Science Easy Science Molar Volume Molar Mass From hu.pinterest.com
Molality Easy Science Easy Science Molar Volume Molar Mass From hu.pinterest.com
What is the mole formula. Answer 1 of 3. To find the grams when you have moles you have to multiply the moles by molar mass of NaCl. We assume you are converting between grams In and mole. How many grams are in 150 moles of KMnO4. One mole of N2 is 28 grams Nitrogen has an atomic weight of 14 and one mole of N2 would be 214 28g How many grams are in 1 mole of KCl.
How many moles is 6 H2O.
1 mole is equal to 1 moles In or 114818 grams. 1 mole is equal to 1 moles Potassium or 390983 grams. So 1 mole of sucrose contains 12 moles of carbon atoms 22 moles of hydrogen atoms and 11 moles of oxygen atoms. How many grams are in 2 moles of water. One mole of KCl is 391 grams K 355 grams Cl. The average mass of one H2O molecule is 1802 amu.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles KMnO4 or 158033949 grams. The number of atoms is an exact number the number of mole is an exact number. 34229648 grams How many moles are there in sucrose. By calculating we get the molecular weight of CO 2801 gmol. How much does 1 mole of gold weigh.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles KMnO4 or 158033949 grams. One mole of H2O is made up of 2 moles of Hydrogen atoms and 1 mole of Oxygen atom. You can view more details on each measurement unit. The number of atoms is an exact number the number of mole is an exact number. N m M where M is the molar mass of this material.
 Source: pinterest.com
Source: pinterest.com
Answer 1 of 3. Here we are given with 16 g of oxygen. Check the chart for more details. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. Since you need to find for 360 mol of H 2 SO 4 360 mol x 106076 gmol 38187 g of H2SO4.
 Source: pinterest.com
Source: pinterest.com
Find the molecular weight of Carbon monoxide. Molecular weight of In or mol. The average mass of one mole of H2O is 1802 grams. 1 grams 1 1 mole using the molecular weight calculator and the molar mass of 1. 1 grams In is equal to 00087094358027487 mole.
 Source: pinterest.com
Source: pinterest.com
The average mass of one H2O molecule is 1802 amu. Find the molecular weight of Carbon monoxide. In other words 1 mole of oxygen would contain molecules. The SI base unit for amount of substance is the mole. You can view more details on each measurement unit.
 Source: pinterest.com
Source: pinterest.com
One mole of H2O is made up of 2 moles of Hydrogen atoms and 1 mole of Oxygen atom. The answer is 2401528. Molecular weight of 6H2O or mol The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Si or 280855 grams. Do a quick conversion.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. The answer should be rounded off to three significant figures resulting in 306 g. Answer 1 of 3. How many grams are in 2 moles of water. Use this page to learn how to convert between moles In and gram.
 Source: pinterest.com
Source: pinterest.com
Find the molecular weight of Carbon monoxide. Use this page to learn how to convert between moles In and gram. To obtain the number of moles divide the mass of compound by the molar mass of the compound expressed in grams. Molecular weight of 6H2O or mol The SI base unit for amount of substance is the mole. Type in your own numbers in the form to convert the units.
 Source: pinterest.com
Source: pinterest.com
How many moles is 6 H2O. We assume you are converting between grams 6H2O and mole. How many grams are in 150 moles of KMnO4. 3 Following step three we obtain. 34229648 grams How many moles are there in sucrose.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles Si or 280855 grams. The SI base unit for amount of substance is the mole. Molecular weight of 6H2O or mol The SI base unit for amount of substance is the mole. 1 grams 6H2O is equal to 0041640155767495 mole. One mole of KCl is 391 grams K 355 grams Cl.
 Source: pinterest.com
Source: pinterest.com
Type in your own numbers in the form to convert the units. To find molecules atoms or ions. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Note that rounding errors may occur so always check the results. Convert 3 moles of carbon monoxide to grams.
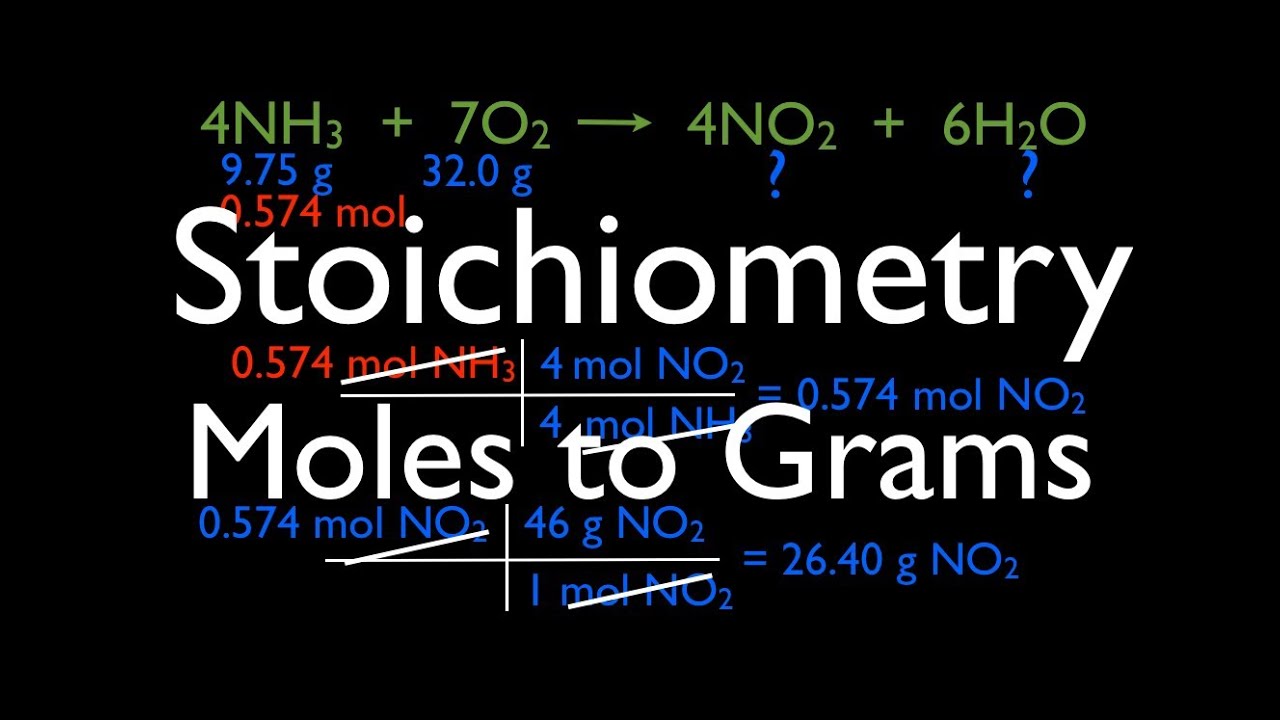 Source: pinterest.com
Source: pinterest.com
You can view more details on each measurement unit. One mole of N2 is 28 grams Nitrogen has an atomic weight of 14 and one mole of N2 would be 214 28g How many grams are in 1 mole of KCl. To obtain the number of moles divide the mass of compound by the molar mass of the compound expressed in grams. Note that rounding errors may occur so always check the results. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles KMnO4 or 158033949 grams. So multiply 1946 by 5844 and you get 11372424grams dont know how far you round. The average mass of one H2O molecule is 1802 amu. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. One mole of H2O is made up of 2 moles of Hydrogen atoms and 1 mole of Oxygen atom.
 Source: hu.pinterest.com
Source: hu.pinterest.com
However one mole of hydrogen atoms has a mass of 1 gram while one MOLE of hydrogen molecules has a mass of 2 grams. However one mole of hydrogen atoms has a mass of 1 gram while one MOLE of hydrogen molecules has a mass of 2 grams. To obtain the number of moles divide the mass of compound by the molar mass of the compound expressed in grams. 3 Following step three we obtain. Check the chart for more details.
 Source: pinterest.com
Source: pinterest.com
1 mole 60221023 6022 10 23 atoms molecules protons etc. 1 mole is equal to 1 moles HNo3 or 77800794 grams. One mole of N2 is 28 grams Nitrogen has an atomic weight of 14 and one mole of N2 would be 214 28g How many grams are in 1 mole of KCl. How much does 1 mole of gold weigh. The number of molecules in 16 gm of oxygen are 05 1632 moles.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in one mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






