Your How many grams are in 1 molecule of o2 images are available. How many grams are in 1 molecule of o2 are a topic that is being searched for and liked by netizens now. You can Get the How many grams are in 1 molecule of o2 files here. Get all royalty-free photos and vectors.
If you’re searching for how many grams are in 1 molecule of o2 images information related to the how many grams are in 1 molecule of o2 interest, you have come to the right blog. Our website always gives you hints for seeking the maximum quality video and image content, please kindly surf and locate more informative video articles and images that fit your interests.
How Many Grams Are In 1 Molecule Of O2. That can react with or replace one gram of hydrogen. Hence 1 g-atom contains 05 Avagadro number of atoms. Was this answer helpful. One mole of oxygen atoms if you could ever isolate them would have a mass of 16 g.
 What Weight Of Oxygen Gas Will Contain The Same Number Of Molecules As 56 G Of Nitrogen Gas Youtube From youtube.com
What Weight Of Oxygen Gas Will Contain The Same Number Of Molecules As 56 G Of Nitrogen Gas Youtube From youtube.com
Now the weight of one oxygen is 16 grams. Recall that two hydrogen atoms bind to make a hydrogen molecule. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 1023 oxygen atoms. One mole of oxygen molecules would therefore have a mass of 32 g. See also how long not long martin luther king. As oxygen exists in diatomic state 1 molecule of oxygen contains 2 atoms of oxygen.
Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 10 23 3012 x 10 23 molecules.
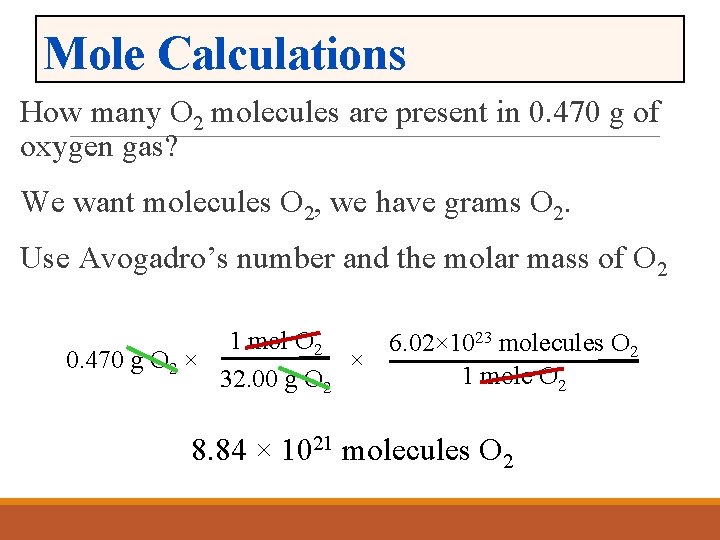
You can view more details on each measurement unit. Mass of O2 molecule 2 16 32 amu. Mass of one molecule of oxygen 32 Avogadro constant 602214076 10 23 531 10-23g. The SI base unit for amount of substance is the mole. Therefore 256 g of oxygen O2 has 963x 1024 atoms of oxygens. Then 1000 g 151001 gmol X g moles.
 Source: youtube.com
Source: youtube.com
Thus 1 mole of oxygen molecules means 6022 x 1023 O2 molecules or 2 x 6022 x 1023 O atoms. The same can be said of one mole of any other substance where two atoms combine to make a simple molecule. How many gram atoms are present in 256g of o2. 1 moles O2 to grams 319988 grams. 7 moles O2 to grams 2239916 grams.

To convert from molecules to grams it is necessary to first convert the number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance. Mass of O2 molecule 2 16 32 amu. 32 Avogadro constant 6023 10 23 531 10 -23 g. Furthermore how many atoms are in 16g of oxygen. To convert from molecules to grams it is necessary to first convert the number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance.
 Source: slidetodoc.com
Source: slidetodoc.com
Thus 1 mole of oxygen molecules means 6022 x 1023 O2 molecules or 2 x 6022 x 1023 O atoms. How many molecules of oxygen O2 are in 16 grams of oxygen O2. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 10 23 3012 x 10 23 molecules. The gram equivalent for oxygen is 8g while a mole of oxygen atoms would be 16g and a mole of oxygen molecules O2 will be 32g. How many grams Oxygen in 1 mol.
 Source: youtube.com
Source: youtube.com
Number of moles 18 g molar mass of H 2 molar mass of O. A molecule of O2 has a mass of 2 16 32 amu. What is the weight of one mole of oxygen molecule. In 1 mole of water there are 6022. The answer is 159994.

These numbers are either in atomic mass units amu or in grams per mole of atoms. Search Results related to mass of o2 in grams on Search Engine. Mass of one molecule of oxygen 32 Avogadro constant 602214076 10 23 531 10-23g. 5 moles O2 to grams 159994 grams. 7 moles O2 to grams 2239916 grams.

1 g-atom 05 mole. For example oxygen gas O 2 is diatomic each molecule contains two atoms so its relative formula mass is 32. Furthermore how many atoms are in 16g of oxygen. Molar Mass Or Molecular Weight Of O2. Number of moles 18 g molar mass of H 2 molar mass of O.
 Source: es.pinterest.com
Source: es.pinterest.com
For example oxygen gas O 2 is diatomic each molecule contains two atoms so its relative formula mass is 32. Then multiply by Avogadros 6022140857 1023 molecules per g mole. Add the two together and you have your formula weight of the molecule equal to 18016 grams. How many gram atoms are present in 256g of o2. Mass of one molecule of oxygen.
 Source: youtube.com
Source: youtube.com
An oxygen atom has a mass of 16 amu. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. The relative molecular mass of oxygen gas is 32 1616 therefore the mass of oxygen in grams 00249 32 07968 grams Also note that. Mass of one molecule of oxygen. If you have 1000 grams.
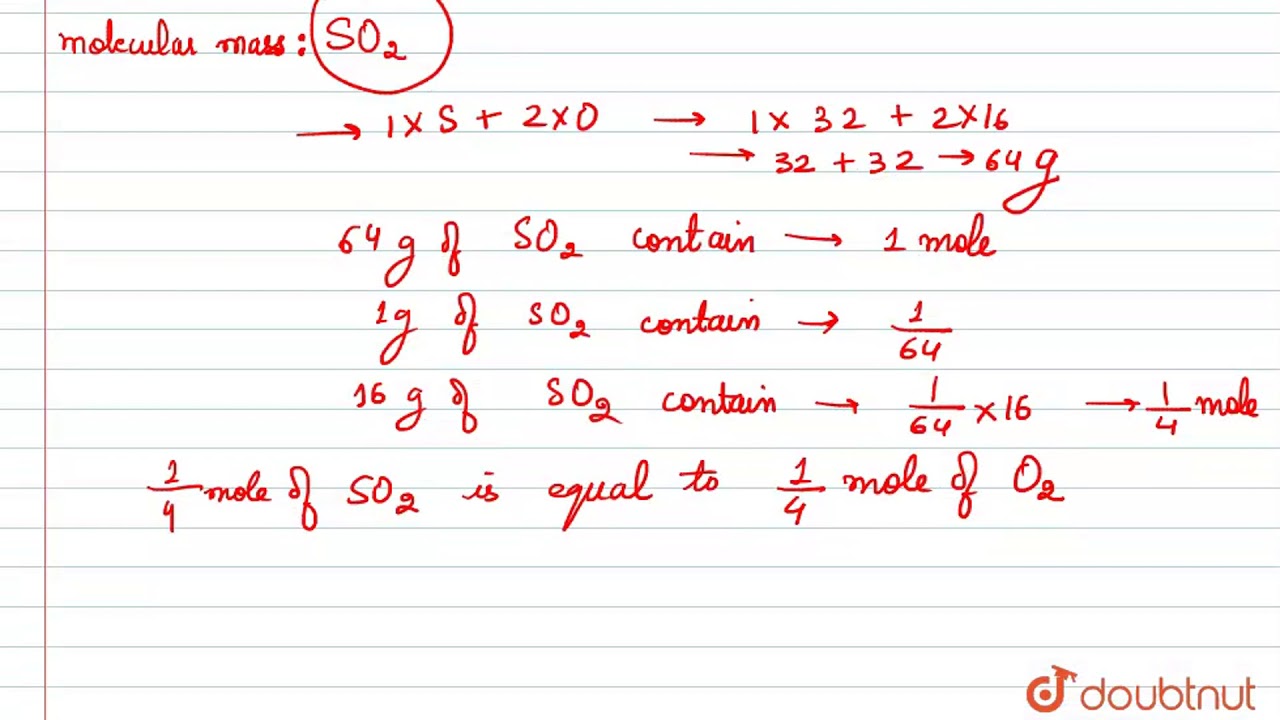 Source: m.youtube.com
Source: m.youtube.com
What is 1g of oxygen. Mass of one molecule of oxygen. How many molecules of oxygen O2 are in 16 grams of oxygen O2. This means that 1 MOLE of hydrogen atoms will weigh 1008 grams. 1 g-atom 05 mole.
One gram molecule represents one mole molecules of oxygen gas. Thus 1 mole of oxygen molecules means 6022 x 1023 O2 molecules or 2 x 6022 x 1023 O atoms. How many gram atoms are present in 256g of o2. Now the weight of one oxygen is 16 grams. Search Results related to mass of o2 in grams on Search Engine.
 Source: toppr.com
Source: toppr.com
In 1 mole of water there are 6022. Mass of O 2 molecule 2 16 32 amu. What Is Molar Mass. 9 moles O2 to grams 2879892 grams. A molecule of O2 has a mass of 2 16 32 amu.

7 moles O2 to grams 2239916 grams. O2 is a diatomic molecule. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. One mole of oxygen molecules would therefore have a mass of 32 g. To convert from molecules to grams it is necessary to first convert the number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance.
 Source: youtube.com
Source: youtube.com
These numbers are either in atomic mass units amu or in grams per mole of atoms. 1 mole of oxygen atoms means 6022 x 1023 O atoms. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 1023 oxygen atoms. Therefore 1 gram molecule of oxygen represents one mole of O2 gas. Then multiply by Avogadros 6022140857 1023 molecules per g mole.

That can react with or replace one gram of hydrogen. 3 moles O2 to grams 959964 grams. 10 23 molecules number of Avogadro. Thus 1 mole of oxygen molecules means 6022 x 1023 O2 molecules or 2 x 6022 x 1023 O atoms. A molecule of O2 has a mass of 2 16 32 amu.
 Source: youtube.com
Source: youtube.com
Molar Mass Or Molecular Weight Of O2. If you have 1000 grams. Search Results related to mass of o2 in grams on Search Engine. The molar mass of O2 is 2 x 1600 or 3200 grams 1 mole O2 3200 grams O2. 8 moles O2 to grams 2559904 grams.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams are in 1 molecule of o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.