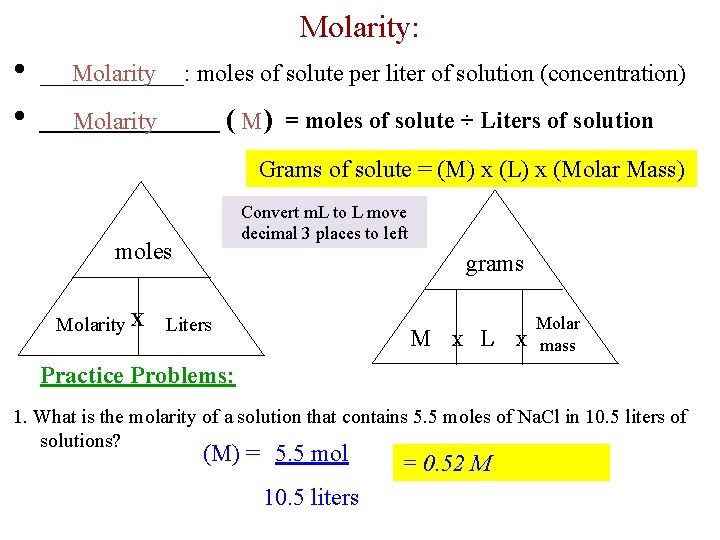
Your How many grams are in 1 mole of o2 images are available in this site. How many grams are in 1 mole of o2 are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams are in 1 mole of o2 files here. Get all royalty-free photos and vectors.
If you’re searching for how many grams are in 1 mole of o2 images information related to the how many grams are in 1 mole of o2 keyword, you have pay a visit to the ideal site. Our website always provides you with hints for refferencing the highest quality video and picture content, please kindly hunt and find more informative video content and graphics that fit your interests.
How Many Grams Are In 1 Mole Of O2. In the decomposition reaction 1 mole of water MW 18015 gmol was produced for every mole of CuO MW 79545 gmol produced. What we need to do is determine an amount of one product either moles or mass assuming all of each reactant reacts. Here we got 88 g of carbon dioxide and we need to convert it into moles. The key to recognizing which reactant is the limiting reactant is based on a mole-mass or mass-mass calculation.
 Day 33 Notes Chapter 7 Chemical Quantities The Mole Ppt Download From slideplayer.com
Day 33 Notes Chapter 7 Chemical Quantities The Mole Ppt Download From slideplayer.com
B What is the partial pressure of O2 in the mixture. Pressure of 150 atm. 8 Using the following equation. 4 NH3 5 O2 —– 4 NO 6 H2O. If 36 g of CuO was produced during the reaction how many grams of water were released as water vapor. What we need to do is determine an amount of one product either moles or mass assuming all of each reactant reacts.
P2 P1V1 150 atm225 L 338 atm is the partial pressure of each gas including O2 V2 100 L PT 3338 atm 101 atm 17.
2 KClO3s 2 KCls 3 O2g. B What is the partial pressure of O2 in the mixture. Calculate the number of moles and the number of grams of iodine I2 that can be made this way from 164 grams of NaIO3. Number of moles mass g molar mass gmol. NaIO3 6 HI —– 3 I2 NaI 3 H2O. Approximately 60 g of C_6H_12O_6.
 Source: meritnation.com
Source: meritnation.com
NaIO3 6 HI —– 3 I2 NaI 3 H2O. If the percent yield for the following reaction is 650 how many grams of KClO3 are needed to produce 420 g of O2. If 36 g of CuO was produced during the reaction how many grams of water were released as water vapor. Number of moles mass g molar mass gmol. P2 P1V1 150 atm225 L 338 atm is the partial pressure of each gas including O2 V2 100 L PT 3338 atm 101 atm 17.
 Source: youtube.com
Source: youtube.com
We have the balanced equation without state symbols. We have the balanced equation without state symbols. 8 Using the following equation. Here we got 88 g of carbon dioxide and we need to convert it into moles. A If all three are forced into the same 100 L container without change in temperature what will be the resulting pressure.
 Source: youtube.com
Source: youtube.com
A If all three are forced into the same 100 L container without change in temperature what will be the resulting pressure. What we need to do is determine an amount of one product either moles or mass assuming all of each reactant reacts. Calculate the number of moles and the number of grams of iodine I2 that can be made this way from 164 grams of NaIO3. 6H_2O6CO_2-C_6H_12O_66O_2 So we would need six moles of carbon dioxide to fully produce one mole of glucose. A If all three are forced into the same 100 L container without change in temperature what will be the resulting pressure.
 Source: youtube.com
Source: youtube.com
B What is the partial pressure of O2 in the mixture. Pressure of 150 atm. If the percent yield for the following reaction is 650 how many grams of KClO3 are needed to produce 420 g of O2. B What is the partial pressure of O2 in the mixture. Approximately 60 g of C_6H_12O_6.
 Source: slideplayer.com
Source: slideplayer.com
P2 P1V1 150 atm225 L 338 atm is the partial pressure of each gas including O2 V2 100 L PT 3338 atm 101 atm 17. If the percent yield for the following reaction is 650 how many grams of KClO3 are needed to produce 420 g of O2. 8 Using the following equation. Calculate the number of moles and the number of grams of iodine I2 that can be made this way from 164 grams of NaIO3. Pressure of 150 atm.
 Source: slideplayer.com
Source: slideplayer.com
We have the balanced equation without state symbols. 6H_2O6CO_2-C_6H_12O_66O_2 So we would need six moles of carbon dioxide to fully produce one mole of glucose. Approximately 60 g of C_6H_12O_6. What we need to do is determine an amount of one product either moles or mass assuming all of each reactant reacts. P2 P1V1 150 atm225 L 338 atm is the partial pressure of each gas including O2 V2 100 L PT 3338 atm 101 atm 17.
 Source: slideplayer.com
Source: slideplayer.com
Carbon dioxide has a molar mass of 44 gmol. 6H_2O6CO_2-C_6H_12O_66O_2 So we would need six moles of carbon dioxide to fully produce one mole of glucose. Pressure of 150 atm. We have the balanced equation without state symbols. B What is the partial pressure of O2 in the mixture.
 Source: youtube.com
Source: youtube.com
Calculate the number of moles and the number of grams of iodine I2 that can be made this way from 164 grams of NaIO3. If the percent yield for the following reaction is 650 how many grams of KClO3 are needed to produce 420 g of O2. Whichever reactant gives the lesser amount of product is the limiting reactant. Here we got 88 g of carbon dioxide and we need to convert it into moles. Carbon dioxide has a molar mass of 44 gmol.
 Source: youtube.com
Source: youtube.com
6H_2O6CO_2-C_6H_12O_66O_2 So we would need six moles of carbon dioxide to fully produce one mole of glucose. 2 KClO3s 2 KCls 3 O2g. In the decomposition reaction 1 mole of water MW 18015 gmol was produced for every mole of CuO MW 79545 gmol produced. If 36 g of CuO was produced during the reaction how many grams of water were released as water vapor. Whichever reactant gives the lesser amount of product is the limiting reactant.
 Source: youtube.com
Source: youtube.com
2 KClO3s 2 KCls 3 O2g. If the percent yield for the following reaction is 650 how many grams of KClO3 are needed to produce 420 g of O2. B What is the partial pressure of O2 in the mixture. Calculate the number of moles and the number of grams of iodine I2 that can be made this way from 164 grams of NaIO3. 8 Using the following equation.

8 Using the following equation. The key to recognizing which reactant is the limiting reactant is based on a mole-mass or mass-mass calculation. Pressure of 150 atm. Approximately 60 g of C_6H_12O_6. In the decomposition reaction 1 mole of water MW 18015 gmol was produced for every mole of CuO MW 79545 gmol produced.
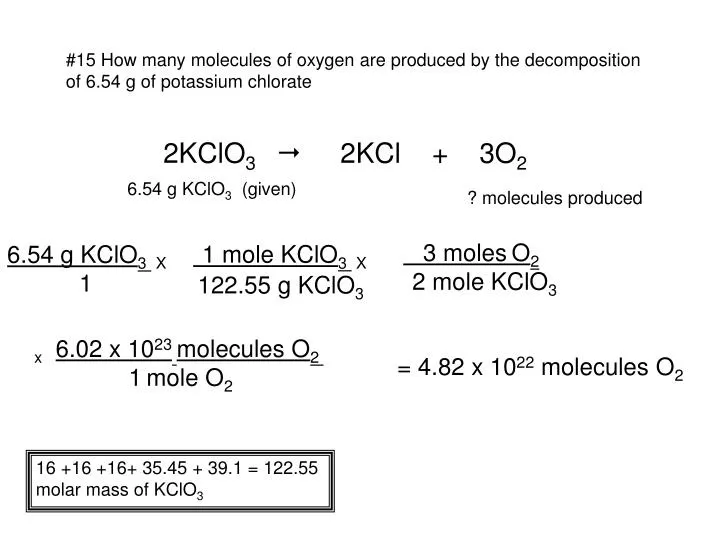 Source: slideserve.com
Source: slideserve.com
8 Using the following equation. Calculate the number of moles and the number of grams of iodine I2 that can be made this way from 164 grams of NaIO3. 6H_2O6CO_2-C_6H_12O_66O_2 So we would need six moles of carbon dioxide to fully produce one mole of glucose. We have the balanced equation without state symbols. B What is the partial pressure of O2 in the mixture.
 Source: toppr.com
Source: toppr.com
2 KClO3s 2 KCls 3 O2g. The key to recognizing which reactant is the limiting reactant is based on a mole-mass or mass-mass calculation. Choose the closest answer. Pressure of 150 atm. Carbon dioxide has a molar mass of 44 gmol.
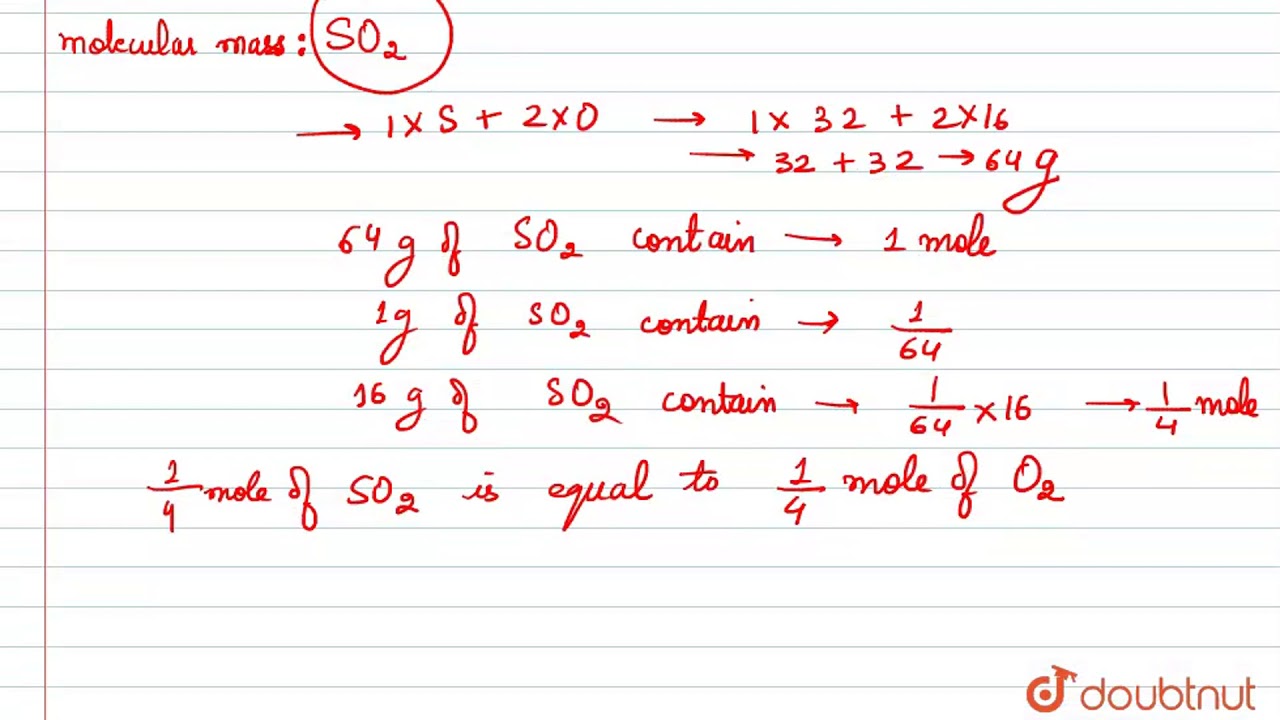 Source: m.youtube.com
Source: m.youtube.com
6H_2O6CO_2-C_6H_12O_66O_2 So we would need six moles of carbon dioxide to fully produce one mole of glucose. Pressure of 150 atm. Number of moles mass g molar mass gmol. B What is the partial pressure of O2 in the mixture. The key to recognizing which reactant is the limiting reactant is based on a mole-mass or mass-mass calculation.
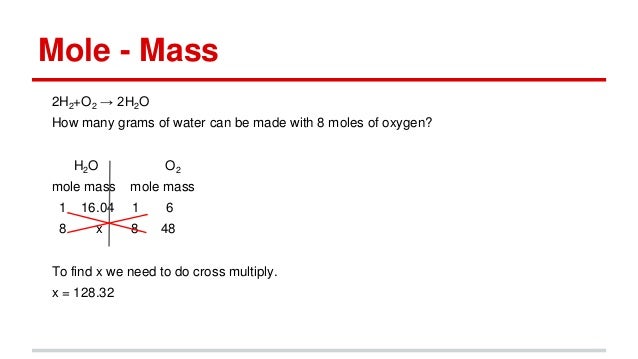 Source: slideshare.net
Source: slideshare.net
Here we got 88 g of carbon dioxide and we need to convert it into moles. Whichever reactant gives the lesser amount of product is the limiting reactant. 4 NH3 5 O2 —– 4 NO 6 H2O. In the decomposition reaction 1 mole of water MW 18015 gmol was produced for every mole of CuO MW 79545 gmol produced. Choose the closest answer.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in 1 mole of o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.