Your How many grams are in 1 mole of naoh images are available. How many grams are in 1 mole of naoh are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams are in 1 mole of naoh files here. Find and Download all free vectors.
If you’re searching for how many grams are in 1 mole of naoh pictures information linked to the how many grams are in 1 mole of naoh interest, you have pay a visit to the ideal site. Our site always gives you hints for viewing the highest quality video and image content, please kindly search and find more informative video articles and graphics that match your interests.
How Many Grams Are In 1 Mole Of Naoh. How many grams of benzoic acid are necessary to react with 2000 mL of the NaOH solution. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles NaOH or 3999711 grams. Note that rounding errors may occur so always check the results.
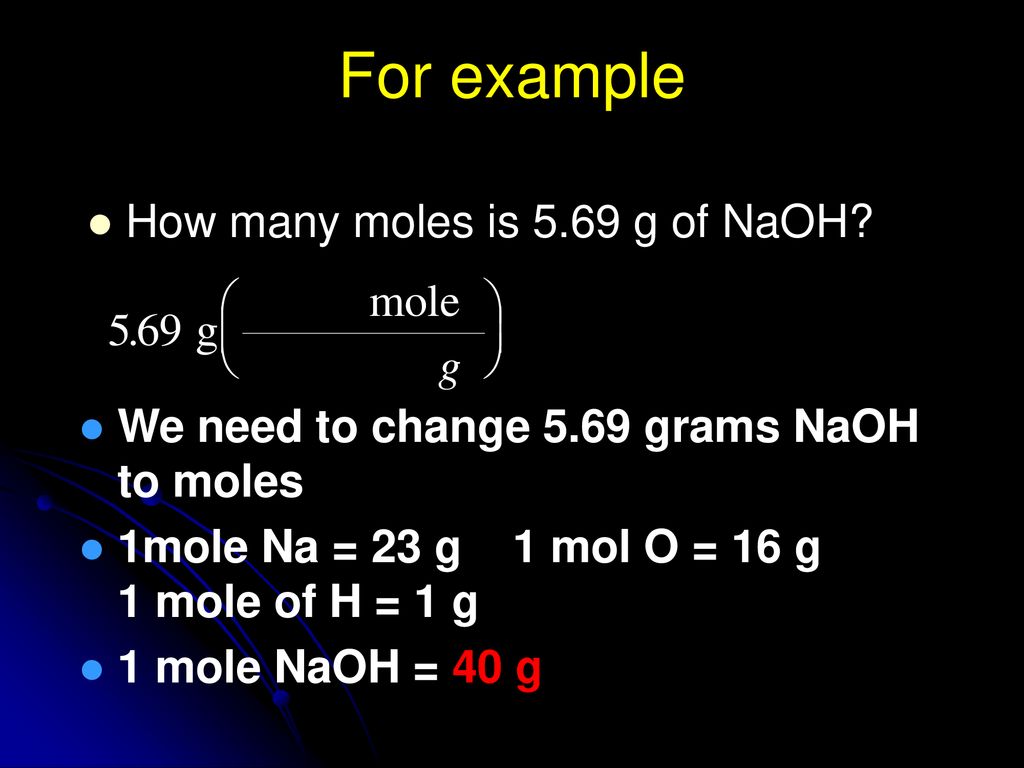 Counting Atoms Chemistry Is A Quantitative Science We Need A Counting Unit The Mole 1 Mole Is The Amount Of Substance That Contains As Many Particles Ppt Download From slideplayer.com
Counting Atoms Chemistry Is A Quantitative Science We Need A Counting Unit The Mole 1 Mole Is The Amount Of Substance That Contains As Many Particles Ppt Download From slideplayer.com
Videos you watch may be added to the TVs watch history and influence TV recommendations. The SI base unit for amount of substance is the mole. 463 x 1024 molecules CCl 4 x 1 mole CCl 4 x 1538 g. 1 grams Sodium Hydroxide is equal to 0025001806380511 mole. The molar mass of benzoic acid is 12212 gmol. Use this page to learn how to convert between grams NaOH and mole.
Since the molar mass of NaOH is 40 gmol we can divide the 90 g of NaOH by the molar mass 40 gmol to find the moles of NaOH.
N 400 mol. In this video well learn to convert moles of NaOH to grams. For our practice problem well. How many moles are in 50 grams of sodium. How many molecules are in 480 grams of NaOH. NaOH molecular weight.

Look at the periodic table. 1 mole is equal to 1 moles Sodium Hydroxide or 3999711 grams. 1 mole is equal to 1 moles KMnO4 or 158033949 grams. The SI base unit for amount of substance is the mole. 1 grams NaOH is equal to 0025001806380511 mole.
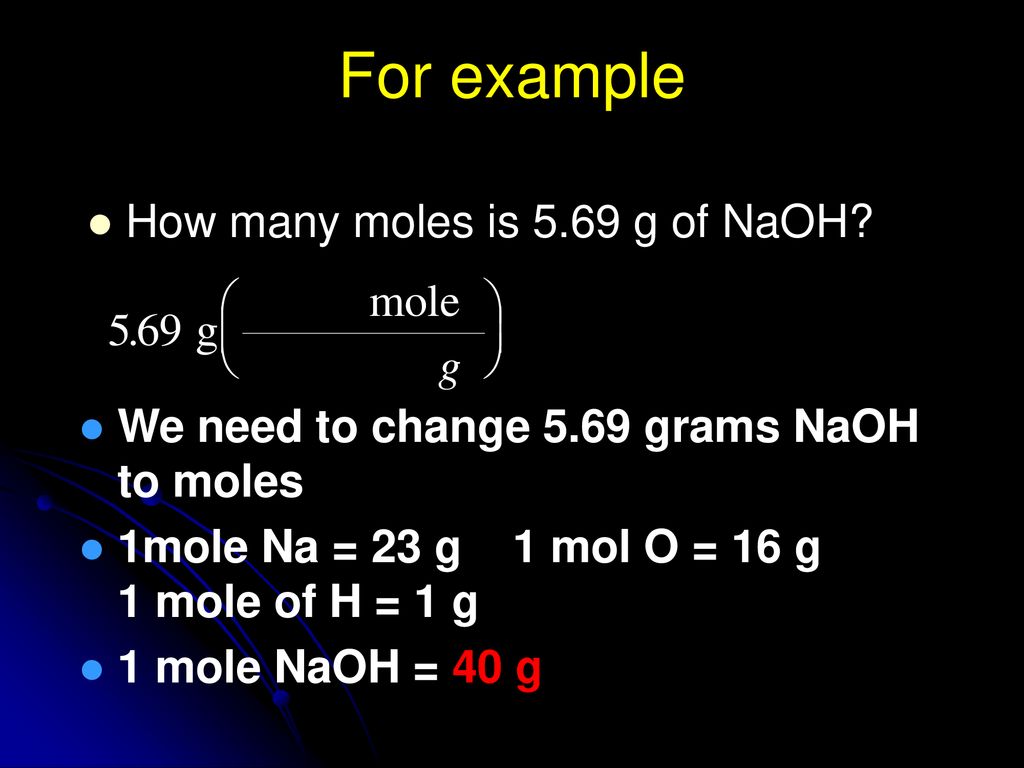 Source: slideplayer.com
Source: slideplayer.com
Type in your own numbers in the form to convert the units. X 1 mole H 3 PO 4 71 x 10-2 moles H 3 PO 4 6022 x 1023 molecules H 3 PO 4 5 How many molecules are in 480 grams of NaOH. Molarity Moles of solute Volume of solution in litres 200 M n 200 L. Look at the periodic table. Molar mass of NaOH 3999711 gmol.
 Source: oneclass.com
Source: oneclass.com
Note that rounding errors may occur so always check the results. X 1 mole H 3 PO 4 71 x 10-2 moles H 3 PO 4 6022 x 1023 molecules H 3 PO 4 5 How many molecules are in 480 grams of NaOH. The answer is 3999711. 400 g H 2 O x 1 mole H 2 chemistry worksheet 6 mixed mole problems grams molecules and liters You now know three things a mole can be. Molecular weight of Sodium Hydroxide or grams.
 Source: clutchprep.com
Source: clutchprep.com
Use this page to learn how to convert between moles NaOH and gram. Note that rounding errors may occur so always check the results. So the molecular weight of NaOH 23161 40. In this video well learn to convert moles of NaOH to grams. Molar mass of NaOH is 40 gmol.
 Source: slideplayer.com
Source: slideplayer.com
Their respective atomic weights are. 1 mole is equal to 1 moles NaOH or 3999711 grams. Convert grams NaOH to moles or moles NaOH to grams. The molecular formula for Sodium Hydroxide is NaOH. Molecular weight of Sodium Hydroxide or grams.
 Source: bartleby.com
Source: bartleby.com
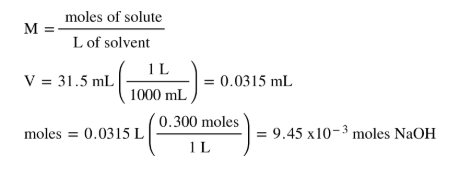
1 mole is equal to 1 moles NaOH or 3999711 grams. Now you know that your solution has a molarity of 0150 M and a volume of 190 mL. The SI base unit for amount of substance is the mole. 400 g H 2 O x 1 mole H 2 chemistry worksheet 6 mixed mole problems grams molecules and liters You now know three things a mole can be. So to prepare 1N solution of NaOH we need to dissolve 40 gms of NaOH in 1000 ml of distilled water.
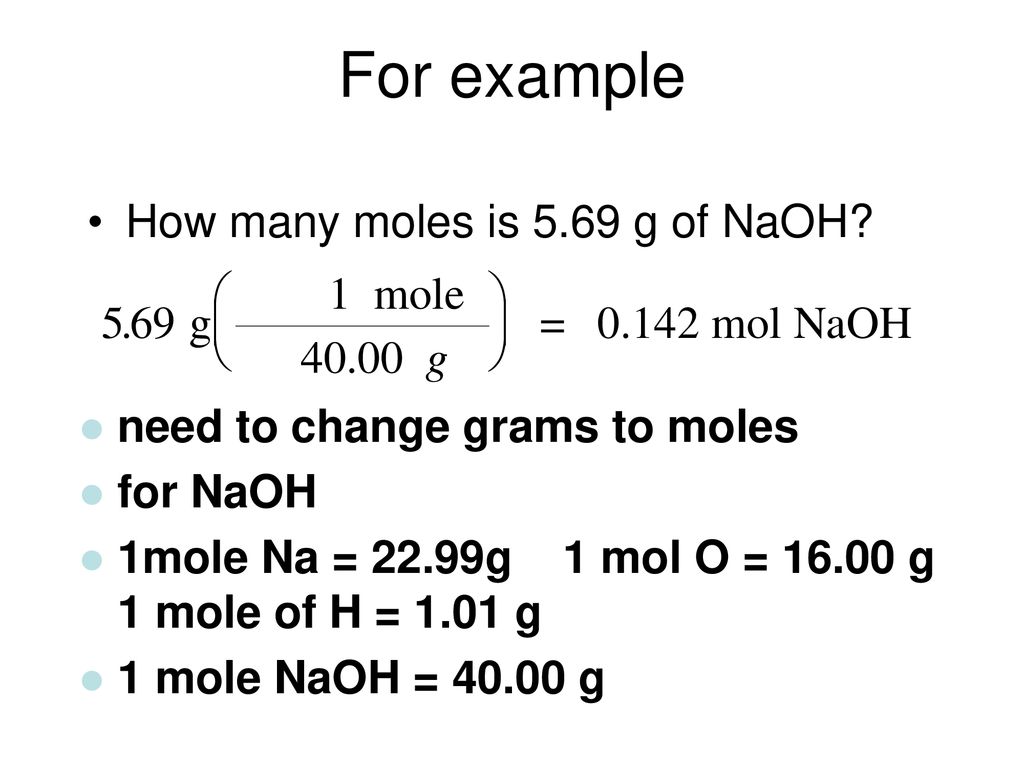 Source: slideplayer.com
Source: slideplayer.com
Na is 23 gmol O is 16 gmol and H is 1 gmol. Molar mass of NaOH is 40 gmol. 1 grams Sodium Hydroxide is equal to 0025001806380511 mole. Type in your own numbers in the form to convert the units. 1 grams NaOH is equal to 0025001806380511 mole.
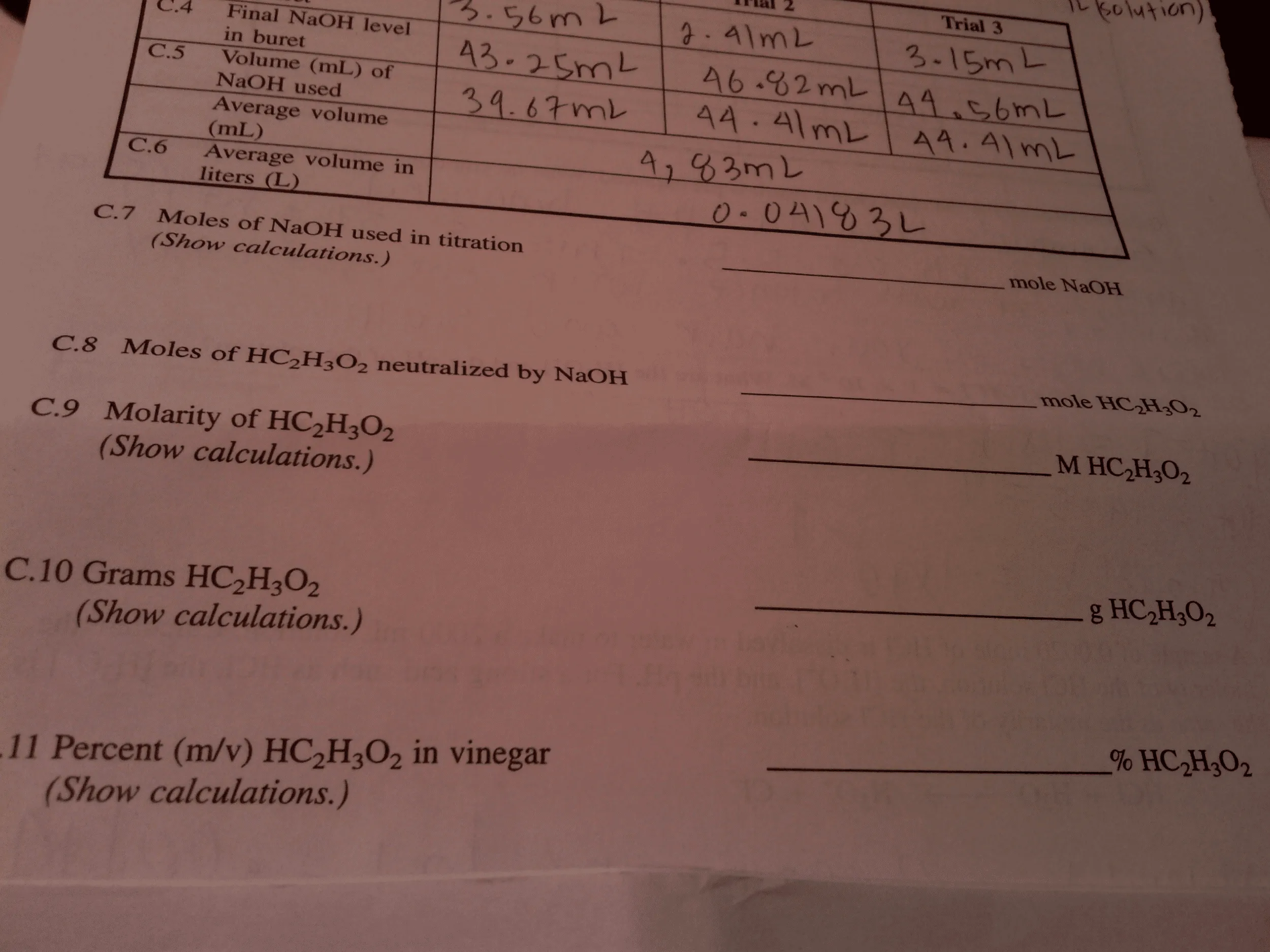 Source: oneclass.com
Source: oneclass.com
You can view more details on each measurement unit. 1 grams Sodium Hydroxide is equal to 0025001806380511 mole. 1 grams naoh 0025001806380511 mole utilizing the molecular weight calculator and the molar mass of naoh. If playback doesnt begin shortly try restarting your device. Since the volume is much smaller than 1 L you can expect to have fewer moles of sodium hydroxide in this sample than you would have had in a full liter.
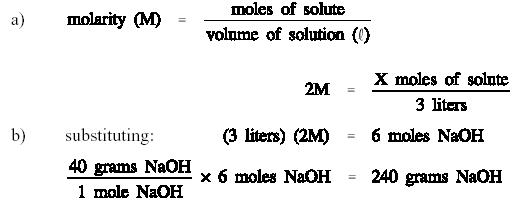 Source: tpub.com
Source: tpub.com
Add all of these up to get the molar mass of NaOH is 40 gmol. Since the molar mass of NaOH is 40 gmol we can divide the 90 g of NaOH by the molar mass 40 gmol to find the moles of NaOH. A chemist makes use of 025 l of 200 m h2so4 to utterly neutralize a 200 l of resolution of naoh. The SI base unit for amount of substance is the mole. If playback doesnt begin shortly try restarting your device.
 Source: youtube.com
Source: youtube.com
The Molarity of a solution is determined by the comparison of the moles of the solute compared to the volume of the solution in liters. N 400 mol. Note that rounding errors may occur so always check the results. 463 x 1024 molecules CCl 4 x 1 mole CCl 4 x 1538 g. 1 mole is equal to 1 moles Sodium Hydroxide or 3999711 grams.
 Source: slideplayer.com
Source: slideplayer.com
How many moles are in 25 grams of NaOH. Molar mass of NaOH 3999711 gmol. 1 grams naoh 0025001806380511 mole utilizing the molecular weight calculator and the molar mass of naoh. NaOH molecular weight. Solutions 1 How many moles are in 400 grams of water.
 Source: youtube.com
Source: youtube.com
Solutions 1 How many moles are in 400 grams of water. 1 mole is equal to 1 moles Sodium Hydroxide or 3999711 grams. Add all of these up to get the molar mass of NaOH is 40 gmol. The technique used can be applied to any mole to gram conversion. The molecular formula for Sodium Hydroxide is NaOH.
 Source: youtube.com
Source: youtube.com
3998 grams NaOH Explanation. Note that rounding errors may occur so always check the results. Now take 120 grams NaOh and multiply this by 1 mol NaOH 40 grams NaOH. A molar mass 602 x 1023 molecules and for. 1 molarity and dilutions issues.
 Source: slideplayer.com
Source: slideplayer.com
The molecular formula for Sodium Hydroxide is NaOH. You can view more details on each measurement unit. 400mol 40 g mol 160 g. Videos you watch may be added to the TVs watch history and influence TV recommendations. How do you convert molar mass to grams.
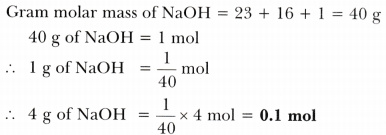 Source: ask.learncbse.in
Source: ask.learncbse.in
SImply put a 1-M solution will have 1 mole of solute dissolved in 1 liter of solution. How do you convert molar mass to grams. To prepare 2N solution of NaOH we must dissolve 24080 gms of NaOH in 1000 ml of distilled water. 2298977 159994 100794 Percent composition by element. Now you know that your solution has a molarity of 0150 M and a volume of 190 mL.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams are in 1 mole of naoh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






