Your How many grams are in 1 mole of mg images are ready. How many grams are in 1 mole of mg are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams are in 1 mole of mg files here. Get all royalty-free images.
If you’re looking for how many grams are in 1 mole of mg images information linked to the how many grams are in 1 mole of mg keyword, you have visit the right blog. Our site always gives you hints for refferencing the maximum quality video and picture content, please kindly hunt and find more enlightening video content and graphics that match your interests.
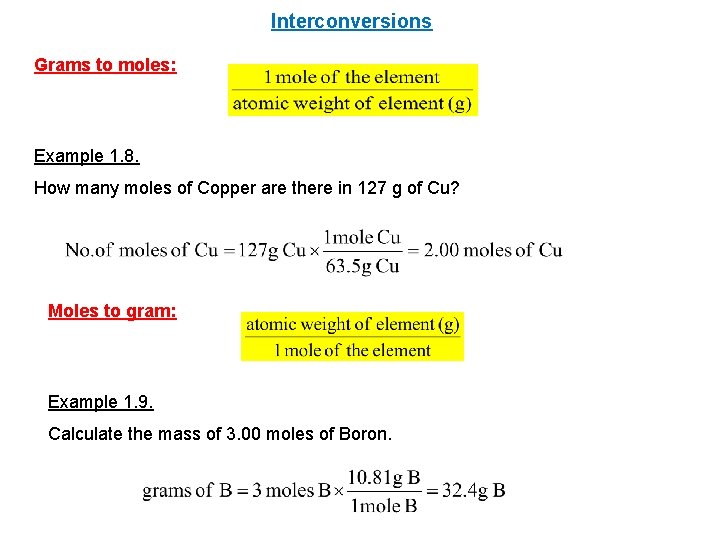
How Many Grams Are In 1 Mole Of Mg. Type in your own numbers in the form to convert the units. Mass of Mg 25g Molar mass of Mg 24gmol Moles of Mg 25g24gmol 1042mol Balance the equation and compare the moles Fe2O3 3Mg 2Fe 3MgO The moles ratio of Mg to Fe is 3. 2 1042 moles of Mg 23 x 1042mol 0695 moles of Fe. The SI base unit for amount of substance is the mole.
 50 Stoichiometry Worksheet Answer Key Chessmuseum Template Library Worksheets Persuasive Writing Prompts Algebra Worksheets From pinterest.com
50 Stoichiometry Worksheet Answer Key Chessmuseum Template Library Worksheets Persuasive Writing Prompts Algebra Worksheets From pinterest.com
To determine the number of moles of each element in 750 mol of the compound multiply the moles of each element times 750. Add the weight of each atom in the compound. 1 mole is equal to 1 moles Magnesium or 24305 grams. Use this page to learn how to convert between moles Mg and gram. Type in your own numbers in the form to convert the units. Mass of 15 mol of MgCl2 is.
In order to convert from milligrams to mmol you will need to make use of the molar mass of the substance in question.
Use this page to learn how to convert between moles Mg and gram. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. Type in your own numbers in the form to convert the units. N2 3H2 – 2NH3 how many grams of hydrogen are needed to react with 50 grams of nitrogen. 5988 kg 5988 g. 1 mole is equal to 1 moles Mg or 24305 grams.
 Source: pinterest.com
Source: pinterest.com
The mass m of the two moles of KOH 2 moles 561056 gmol 1122112 grams The amount in grams of KOH that react with one mole of MgCl₂ 1122112 grams 112 grams 256 g. What is the molar mass of Ca NO3 2. Secondly how many grams are in 238 moles of arsenic. How many atoms are in one mole of magnesium. 485 14507 Views.
 Source: pinterest.com
Source: pinterest.com
Type in your own numbers in the form to convert the units. Note that rounding errors may occur so always check the results. 1 grams Mg is equal to 0041143797572516 mole. Type in your own numbers in the form to convert the units. Secondly how many grams are in 238 moles of arsenic.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles Mg or 24305 grams. You do these problems in steps. However one mole of magnesium weighs 2431 g. To get the mass of Mg you need to multiply the moles of mg123 and the molar mass amu of mg 2430 Ps. 5988 kg 5988 g.
 Source: pinterest.com
Source: pinterest.com
Its easier to work with grams so convert the mass. How many atoms are there in 05 moles. What is the molar mass of Ca NO3 2. Type in your own numbers in the form to convert the units. However one mole of magnesium weighs 2431 g.
 Source: pinterest.com
Source: pinterest.com
1 mol MgClO42 contains 1 mol Mg atoms2 mol Cl atomsand8 mol O atoms. The figure means that the mass of one mole of Mg is 243g. 1 mol MgClO42 contains 1 mol Mg atoms2 mol Cl atomsand8 mol O atoms. To get the mass of Mg you need to multiply the moles of mg123 and the molar mass amu of mg 2430 Ps. Molar mass of MgCl2 952 gmol.
 Source: pinterest.com
Source: pinterest.com
5988 kg 5988 g. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. Have to find the moles of mg. 26 Votes This is the weight in grams of 1 mole of the compound. Similarly you may ask how do you convert grams to millimoles.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles Magnesium or 24305 grams. However one mole of magnesium weighs 2431 g. To get the mass of Mg you need to multiply the moles of mg123 and the molar mass amu of mg 2430 Ps. Use this page to learn how to convert between moles Mg and gram. Use this page to learn how to convert between grams Mg and mole.
 Source: pinterest.com
Source: pinterest.com
The mass m of the two moles of KOH 2 moles 561056 gmol 1122112 grams The amount in grams of KOH that react with one mole of MgCl₂ 1122112 grams 112 grams 256 g. Use this page to learn how to convert between grams Mg ClO42 and mole. 179 moles is 50 grams. Have to find the moles of mg. Mass of 15 mol of MgCl2 is.
 Source: pinterest.com
Source: pinterest.com
How many moles of N2 is 50 grams. Mass of Mg 25g Molar mass of Mg 24gmol Moles of Mg 25g24gmol 1042mol Balance the equation and compare the moles Fe2O3 3Mg 2Fe 3MgO The moles ratio of Mg to Fe is 3. You do these problems in steps. Note that rounding errors may occur so always check the results. Use this page to learn how to convert between moles Mg and gram.
 Source: pinterest.com
Source: pinterest.com
Molecular weight of Magnesium or grams The molecular formula for Magnesium is Mg. You can view more details on each measurement unit. However one mole of magnesium weighs 2431 g. 1 mol of Mg has 602214129 10 23 Mg atoms with a molar mass of 24305 gmol. Moles were planned that way Since one mole of MgCl2 consists of one mole of magnesium and two moles of chlorine the mass of one mole of MgCl2 must be the sum of the masses of the elements.
 Source: pinterest.com
Source: pinterest.com
Mass of 15 mol of MgCl2 is. N2 3H2 – 2NH3 how many grams of hydrogen are needed to react with 50 grams of nitrogen. Note that rounding errors may occur so always check the results. Have to find the moles of mg. What is the molar mass of Ca NO3 2.
 Source: pinterest.com
Source: pinterest.com
Mass of Mg 25g Molar mass of Mg 24gmol Moles of Mg 25g24gmol 1042mol Balance the equation and compare the moles Fe2O3 3Mg 2Fe 3MgO The moles ratio of Mg to Fe is 3. However one mole of magnesium weighs 2431 g. Accordingly how many grams are in a. So you divide 7431022 by 60221022 you will get 123 moles of mg. Note that rounding errors may occur so always check the results.
 Source: es.pinterest.com
Source: es.pinterest.com
So you divide 7431022 by 60221022 you will get 123 moles of mg. Similarly you may ask how do you convert grams to millimoles. However one mole of magnesium weighs 2431 g. 5988 kg 5988 g. Molecular weight of Magnesium or grams The molecular formula for Magnesium is Mg.
 Source: pinterest.com
Source: pinterest.com
Molecular weight of Magnesium or grams The molecular formula for Magnesium is Mg. 1 mole is equal to 1 moles Mg or 24305 grams. 1 mol of Mg has 602214129 10 23 Mg atoms with a molar mass of 24305 gmol. 179 moles is 50 grams. To get the mass of Mg you need to multiply the moles of mg123 and the molar mass amu of mg 2430 Ps.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles Magnesium or 24305 grams. Note that rounding errors may occur so always check the results. What is the molar mass of Ca NO3 2. Mass of Mg 25g Molar mass of Mg 24gmol Moles of Mg 25g24gmol 1042mol Balance the equation and compare the moles Fe2O3 3Mg 2Fe 3MgO The moles ratio of Mg to Fe is 3. 15mol 952 g mol 1428 g.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in 1 mole of mg by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.