Your How many grams are in 1 mole of magnesium images are ready. How many grams are in 1 mole of magnesium are a topic that is being searched for and liked by netizens today. You can Download the How many grams are in 1 mole of magnesium files here. Download all royalty-free photos and vectors.
If you’re looking for how many grams are in 1 mole of magnesium pictures information connected with to the how many grams are in 1 mole of magnesium interest, you have come to the ideal site. Our site frequently provides you with suggestions for downloading the highest quality video and picture content, please kindly surf and find more informative video articles and graphics that fit your interests.
How Many Grams Are In 1 Mole Of Magnesium. Avogadros number units or in this case atoms. When you look at a period table the mass thats given for each element is the mass of one mole of that element. 1 Mole of magnesium 24305 grams The 24305 comes from the periodic table of elements. For example one atom of magnesium weighs 2431 amu atomic mass units.
 Unit Conversion Example Moles Of Magnesium Mg In The World S Seawater Youtube From youtube.com
Unit Conversion Example Moles Of Magnesium Mg In The World S Seawater Youtube From youtube.com
Use this page to learn how to convert between moles Magnesium Fluoride and gram. How many grams are in moles. Take the number of grams and divide it by the Atomic Mass. In the question the given mass of magnesium oxide MgO is given as 425grams. From the periodic table we come to know that. Use this page to learn how to convert between moles Magnesium Oxide and gram.
The SI base unit for amount of substance is the mole.
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Magnesium Oxide or 403044 grams. We assume you are converting between grams Magnesium and mole. 1 mole of magnesium 243 gm. What is the mass of 170 mol of carbon 12. Note that rounding errors may occur so always check the results.
 Source: toppr.com
Source: toppr.com
1 mole is equal to 1 moles Magnesium Oxide or 403044 grams. The SI base unit for amount of substance is the mole. What is the mass of 170 mol of carbon 12. Molecular weight of Magnesium or grams. The SI base unit for amount of substance is the mole.
 Source: brainly.com
Source: brainly.com
Magnesiums molar mass is 24305 gmol. 8 How many grams are in 238 moles of arsenic. There are three mole equalities. A mole is quantified as 6022 x 1023 you guessed it. Magnesiums molar mass is 24305 gmol.
 Source: toppr.com
Source: toppr.com
5 How many moles are in 23 grams of phosphorus. As atoms of magnesium present in 2430 gram of magnesium. Moles were planned that way Since one mole of MgCl2 consists of one mole of magnesium and two moles of chlorine the mass of one mole of MgCl2 must be the sum of the masses of the elements. How many grams of magnesium hydroxide are produced when 800 grams of magnesium chloride reacts with excess potassium hydroxide. From the periodic table we come to know that.
 Source: youtube.com
Source: youtube.com
So atoms of magnesium present in gram of magnesium. When you look at a period table the mass thats given for each element is the mass of one mole of that element. Note that rounding errors may occur so always check the results. However one mole of magnesium weighs 2431 g. Use this page to learn how to convert between grams Magnesium and mole.
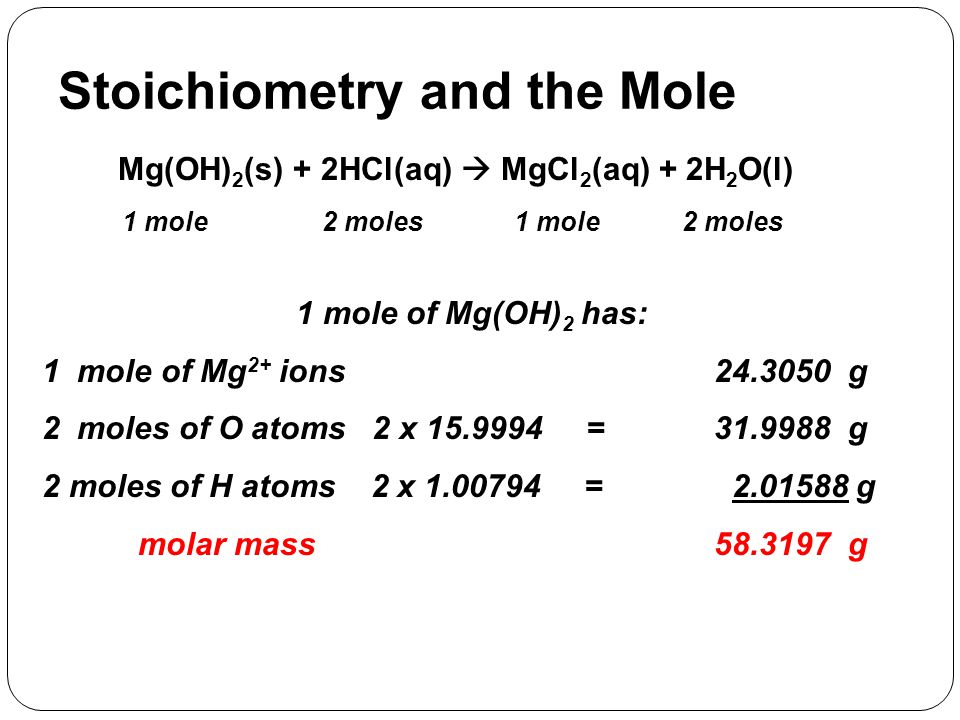 Source: slideplayer.com
Source: slideplayer.com
5 How many moles are in 23 grams of phosphorus. Grams 58443 5 292215 g Molar mass of AgNO3 is 169873 2 kg AgNO3 is equal to how many moles. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. The SI base unit for amount of substance is the mole. 1 mol g-formula-mass periodic table 1 mol 224 L for a gas at STP.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles Magnesium Sulfide or 5637 grams. Use this page to learn how to convert between moles Magnesium Fluoride and gram. Each equality can be written as a set of two conversion factors. 1 grams Magnesium is equal to 0041143797572516 mole. What are the molecular weights of the following compounds.

In the question the given mass of magnesium oxide MgO is given as 425grams. 6 How many grams are in 119 moles of chromium 7 How many moles are in 98 grams of calcium. The SI base unit for amount of substance is the mole. 4 How many grams are in 881 moles of magnesium. For example one atom of magnesium weighs 2431 amu atomic mass units.

The SI base unit for amount of substance is the mole. So atoms of magnesium present in gram of magnesium. Multiply by one mole for units to cancel. The SI base unit for amount of substance is the mole. Grams 58443 5 292215 g Molar mass of AgNO3 is 169873 2 kg AgNO3 is equal to how many moles.

8 How many grams are in 238 moles of arsenic. The mass of magnesium is 299 grams. As atoms of magnesium present in 2430 gram of magnesium. Note that rounding errors may occur so always check the results. 301 x 1022 atoms 5 x 10-2 moles.
 Source: youtube.com
Source: youtube.com
1 grams Magnesium is equal to 0041143797572516 mole. As atoms of magnesium present in 2430 gram of magnesium. 52562 grams 5 g 12 How many moles are in 68 grams of copper II hydroxide CuOH 2. The molar mass of the elements is given in grams per mole. When you look at a period table the mass thats given for each element is the mass of one mole of that element.
 Source: slideplayer.com
Source: slideplayer.com
A mole is quantified as 6022 x 1023 you guessed it. 1 grams Magnesium is equal to 0041143797572516 mole. How many grams are in moles. Each equality can be written as a set of two conversion factors. 1 mol g-formula-mass periodic table 1 mol 224 L for a gas at STP.
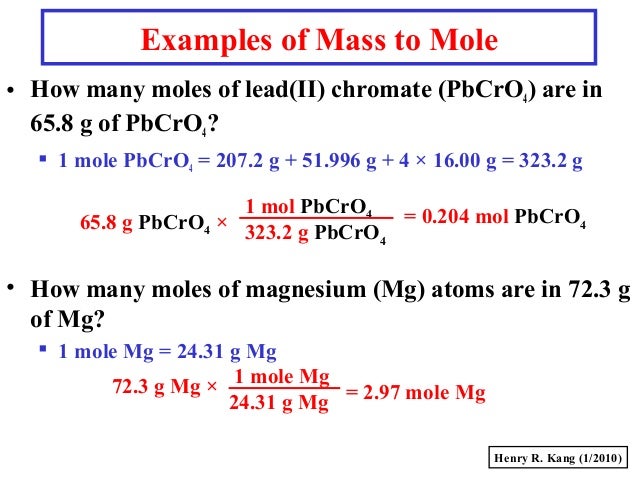 Source: slideshare.net
Source: slideshare.net
1 mole is equal to 1 moles Magnesium Oxide or 403044 grams. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. The SI base unit for amount of substance is the mole. 4 How many grams are in 881 moles of magnesium. And 1 mole of magnesium has 2430 g.
 Source: studylib.net
Source: studylib.net
Avogadros number units or in this case atoms. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. One mole of magnesium weighs 24 grams. So lets find how many moles is 6 grams. Use this page to learn how to convert between moles Magnesium Oxide and gram.
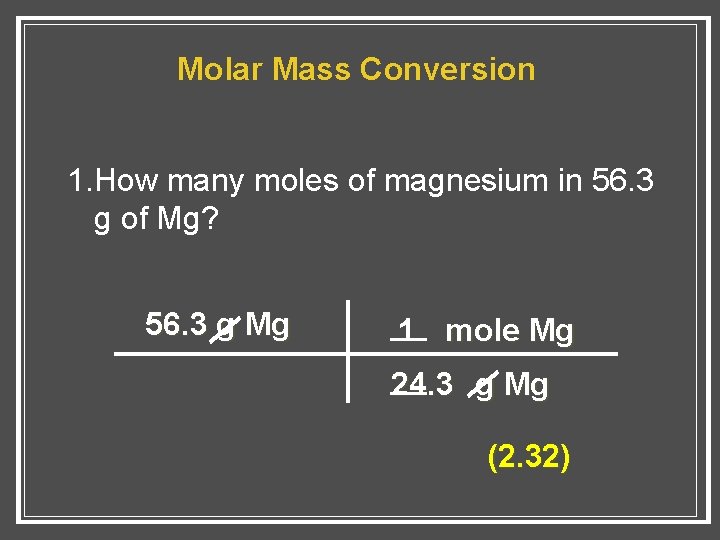 Source: slidetodoc.com
Source: slidetodoc.com
Use this page to learn how to convert between moles Magnesium Oxide and gram. Use this page to learn how to convert between moles Magnesium Fluoride and gram. 1 mole is equal to 1 moles Magnesium Fluoride or 623018064 grams. The molecular formula for Magnesium is Mg. Use this page to learn how to convert between grams Magnesium and mole.
 Source: youtube.com
Source: youtube.com
1 Mole of magnesium 24305 grams The 24305 comes from the periodic table of elements. We have 025 moles soo we have 025 avagadro number. Use this page to learn how to convert between moles Magnesium Oxide and gram. 12601 moles 126 moles 11 How many grams are in 002 moles of beryllium iodide BeI 2. 1 mole of magnesium 243 gm.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams are in 1 mole of magnesium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






