Your How many grams are in 1 mole of hcl images are available in this site. How many grams are in 1 mole of hcl are a topic that is being searched for and liked by netizens now. You can Get the How many grams are in 1 mole of hcl files here. Get all royalty-free vectors.
If you’re looking for how many grams are in 1 mole of hcl pictures information connected with to the how many grams are in 1 mole of hcl keyword, you have pay a visit to the ideal blog. Our website frequently gives you suggestions for seeing the highest quality video and picture content, please kindly surf and locate more enlightening video content and images that match your interests.
How Many Grams Are In 1 Mole Of Hcl. The SI base unit for amount of substance is the mole. Number of moles mass molecular weight. Correct option is D M o l e s m o l e c u l a r w e i g h t w e i g h t Moles of HCl 3 6 5 3 6 5 0 1. How many grams are in 153 moles of HCl Other questions on the subject.
 How Many Moles Of Hcl Are Present In 1 Litre Of 1 M Hcl Solution Youtube From youtube.com
How Many Moles Of Hcl Are Present In 1 Litre Of 1 M Hcl Solution Youtube From youtube.com
First get moles hcl then get the grams hclmolarity moles of soluteliters of solution38 m hcl x moles172 liters 6536 moles hclso6536 moles hcl. We assume you are converting between grams HCl and mole. Check all that apply. N 1 molL x 2 L 2 moles The molar mass of HCl is 3645 gmol. To prepare 1 L of 05 N HCl solution you have to take 05 gram equivalent of pure HCl. 1 mole 60221023 6022 10 23 atoms molecules protons etc.
Molecular weight of HCl or grams This compound is also known as Hydrochloric Acid.
Moles of water 1 8 1 6 2 0 9. Number of moles mass molecular weight. Grams and divide it by the molecular weight to convert to moles. Really what youre doing is multiplying the number by 1 mole and dividing it by the equivalent of one mole the molecular weight. 1N HCL - add 3646 gm of HCL in 1 litre of water Molar mass of HCL 3646 gmole 3646 g of HCL is equivalent to 3067 ml of HCL Volume MassDensity 3646119 3067 Density of HCL is 119 gml. If we have 5g of HCl then we can say that it has 0137 moles.
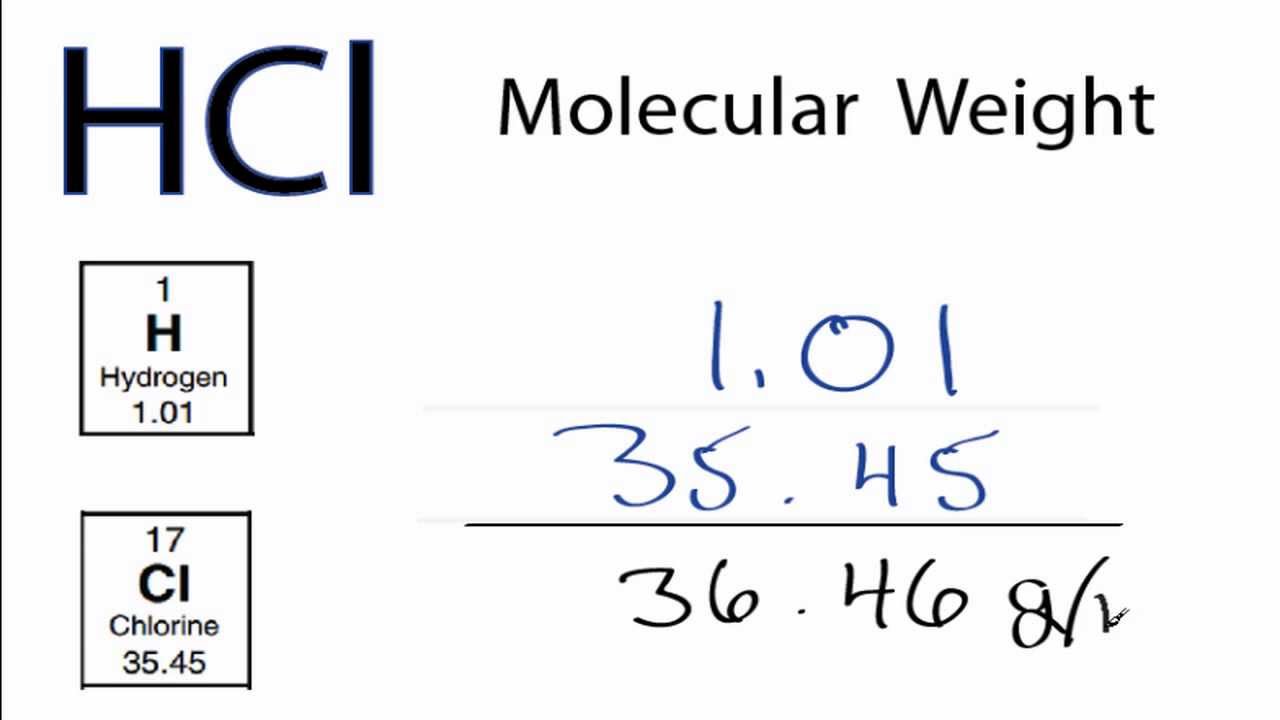 Source: youtube.com
Source: youtube.com
What is the mole equation. We assume you are converting between grams HCl and mole. X 34 x 10²⁴ 1 6022 10²³ 56 moles of HCl. Multiplying this to the number of moles will give us the mass of HCl. 1N HCL - add 3646 gm of HCL in 1 litre of water Molar mass of HCL 3646 gmole 3646 g of HCL is equivalent to 3067 ml of HCL Volume MassDensity 3646119 3067 Density of HCL is 119 gml.
 Source: nagwa.com
Source: nagwa.com
The SI base unit for amount of substance is the mole. Mass of HCl 3645 2 mol 729 grams HCl Advertisement Survey Did this page answer your question. The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results. You can view more details on each measurement unit.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass HCl 3646gmol The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. If we have 5g of HCl then we can say that it has 0137 moles. What are the advantages and disadvantages of nuclear power. Mass number of moles molecular weight. The answer is 0027426610504282.
 Source: wou.edu
Source: wou.edu
1 mole is equal to 1 moles HCl or 3646094 grams. X - the dots are for alignment 1. Grams and divide it by the molecular weight to convert to moles. Molecular weight of HCl or grams This compound is also known as Hydrochloric Acid. 1000mL 1191 g 1mL 1191 g.
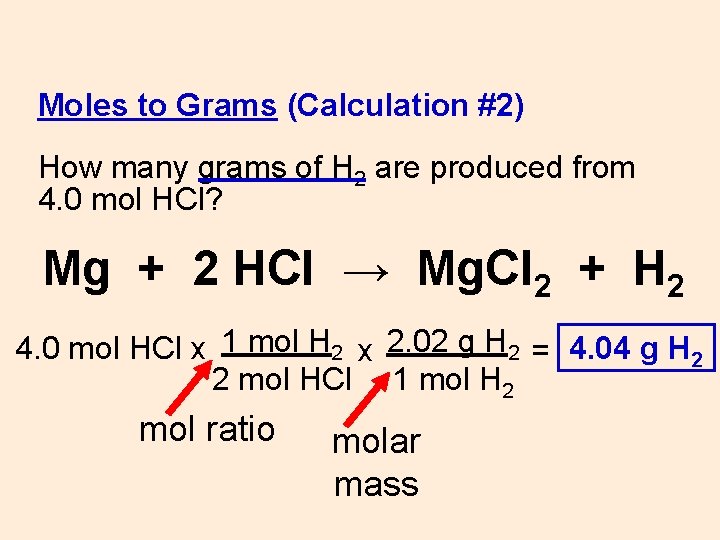 Source: slidetodoc.com
Source: slidetodoc.com
Chemistry 22062019 0600 drma1084. This means that your sample will contain. Thus the number of moles of hydrogen chloride HCl is 124 moles. H 1 Cl 355 HCl 365 gmol. You can view more details on each measurement unit.
 Source: slideplayer.com
Source: slideplayer.com
Moles of water 1 8 1 6 2 0 9. The SI base unit for amount of substance is the mole. So 05 gram equivalent HCl 05 mole HCl 05 x 365 gram HCl 1825 g HCl. Mass of HCl 3645 2 mol 729 grams HCl Advertisement Survey Did this page answer your question. Molar mass HCl 3646gmol The molar mass of any substance is the mass in grams of one mole of representative particles of that substance.
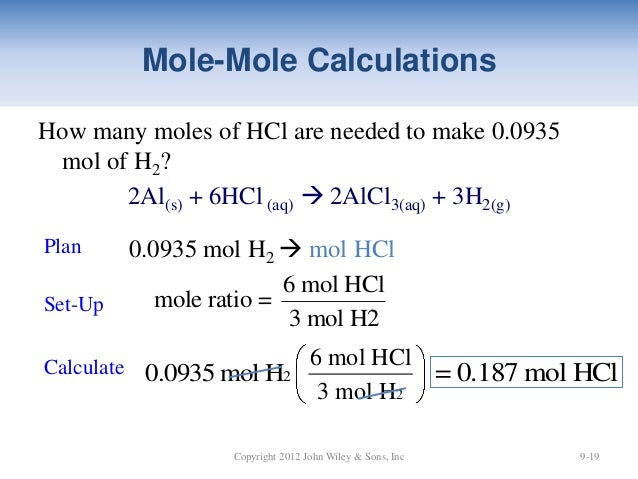 Source: slideshare.net
Source: slideshare.net
Use this page to learn how to convert between moles HCl and gram. So 05 gram equivalent HCl 05 mole HCl 05 x 365 gram HCl 1825 g HCl. Not at all Slightly Kinda. DrBob222 Nov 13 2012 Respond to this Question Similar Questions Chemistry. Molecular weight of HCl or grams This compound is also known as Hydrochloric Acid.
 Source: youtube.com
Source: youtube.com
What is the mole equation. Use this page to learn how to convert between moles HCl and gram. The number of moles in 1 gram of HCl is 36461. 365 grams of HCl is dissolved in 162 grams of water. X - the dots are for alignment 1.
 Source: youtube.com
Source: youtube.com
1 gram equivalent of HCl 1 mole of HCl. Mole fraction of HCl Total moles. Really what youre doing is multiplying the number by 1 mole and dividing it by the equivalent of one mole the molecular weight. How many grams are in 1 mole of HCl. Molecular weight of HCl or grams This compound is also known as Hydrochloric Acid.
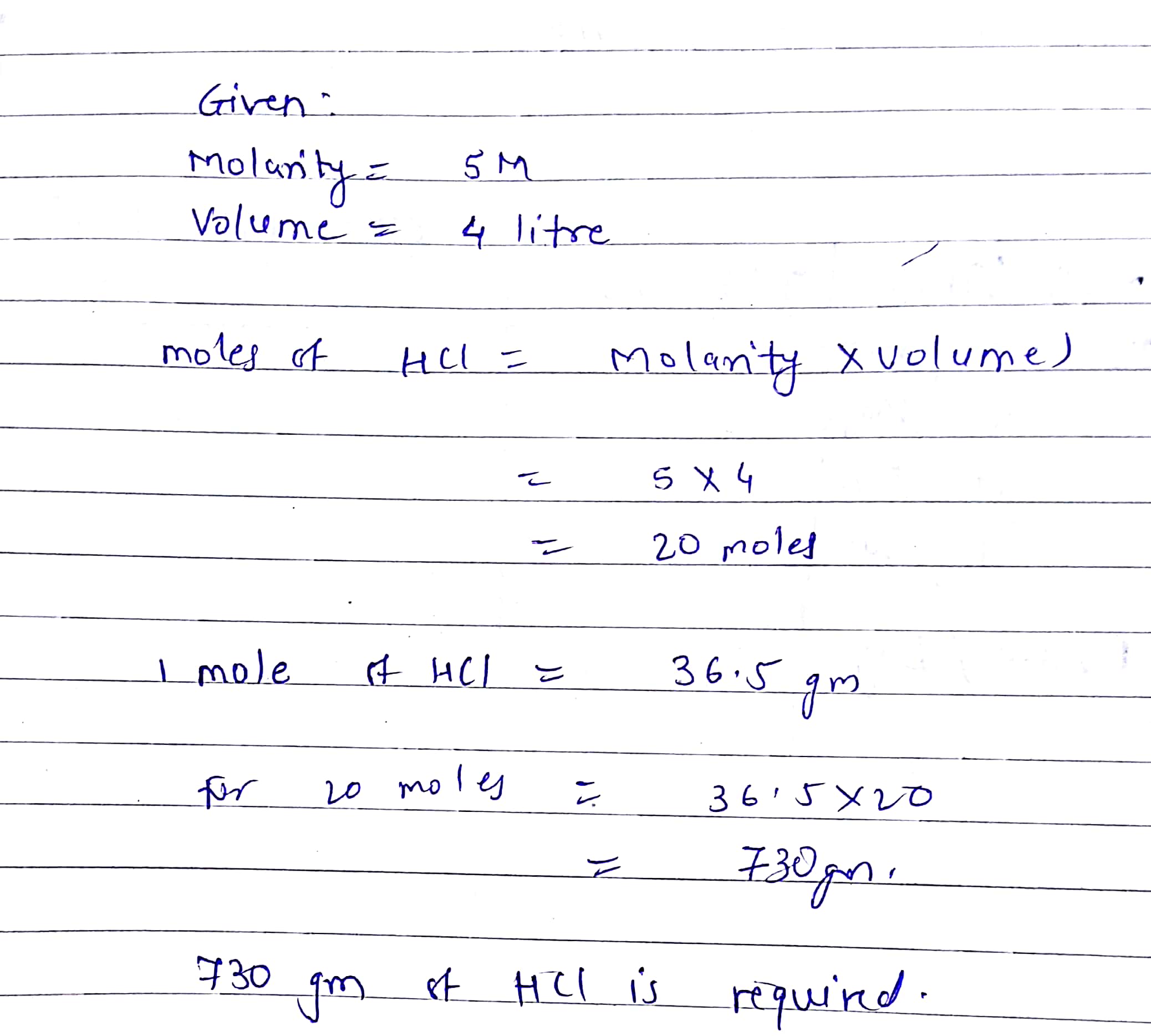 Source: topperlearning.com
Source: topperlearning.com
The gram formula mass of water is 18Each H has a mass of 1 and one O has a mass of 16 and that number also represents the number of grams in 1 mole. To prepare 1 L of 05 N HCl solution you have to take 05 gram equivalent of pure HCl. Ka for HCN 41 10-10. X 34 x 10²⁴ 1 6022 10²³ 56 moles of HCl. Note that rounding errors may occur so always check the results.
 Source: slideplayer.com
Source: slideplayer.com
The mass of the sample you have here will thus be. If there are 6022 10²³ units in 1 mole of Al₂O₃. The mass of the sample you have here will thus be. The SI base unit for amount of substance is the mole. X 259 x 10²³ 1 6022 10²³ 043 moles of Al₂O₃.
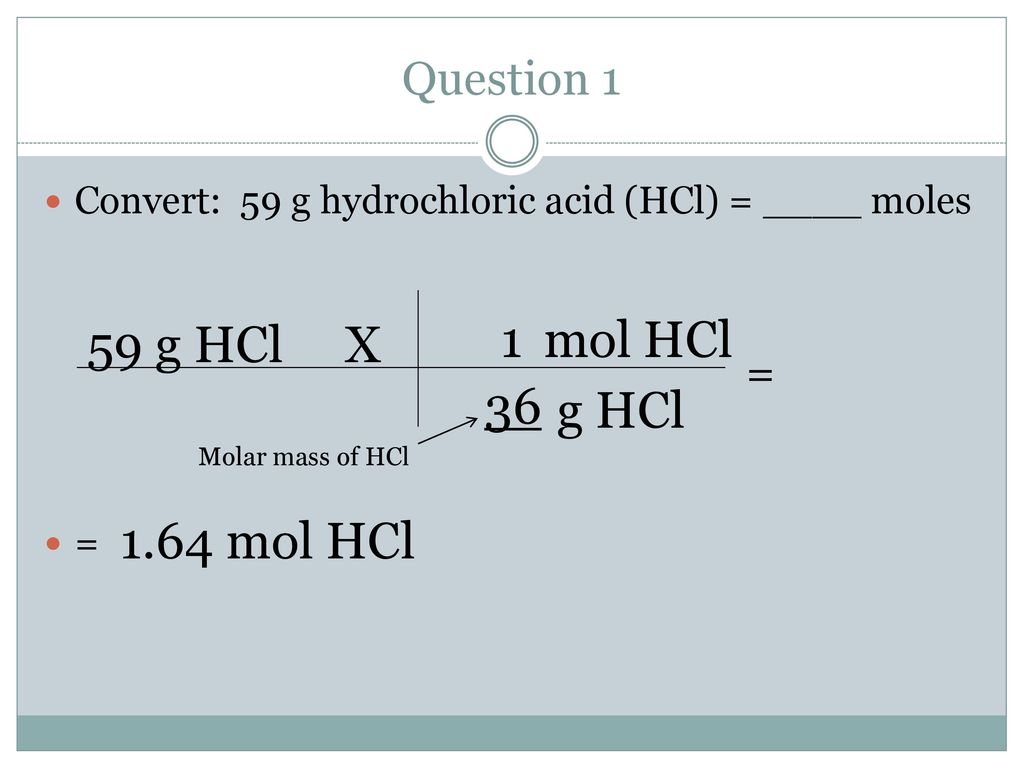 Source: slideplayer.com
Source: slideplayer.com
Moles of water 1 8 1 6 2 0 9. First get moles hcl then get the grams hclmolarity moles of soluteliters of solution38 m hcl x moles172 liters 6536 moles hclso6536 moles hcl. 1000mL 1191 g 1mL 1191 g. X 259 x 10²³ 1 6022 10²³ 043 moles of Al₂O₃. X - the dots are for alignment 1.
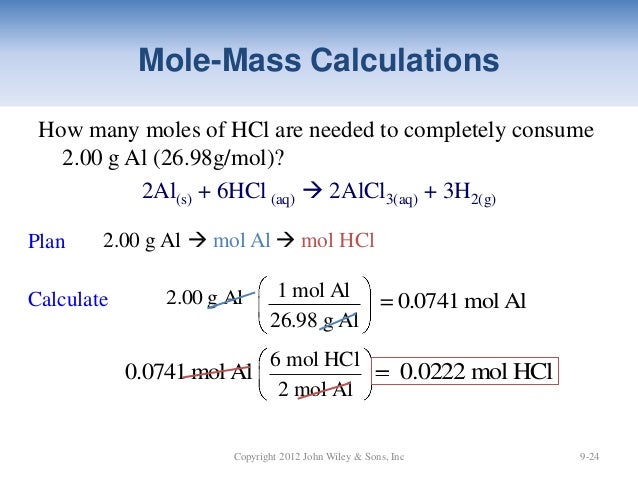 Source: slideshare.net
Source: slideshare.net
1 gram equivalent of HCl 1 mole of HCl. How many grams are in 1 mole of HCl. Mole Given mass Molar mass 452 grams 364 gramsmol 124 mol. 1000mL 1191 g 1mL 1191 g. Use this page to learn how to convert between moles HCl and gram.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. 365 grams of HCl is dissolved in 162 grams of water. Molar mass HCl 3646gmol The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. 1 mole 60221023 6022 10 23 atoms molecules protons etc. Correct option is D M o l e s m o l e c u l a r w e i g h t w e i g h t Moles of HCl 3 6 5 3 6 5 0 1.
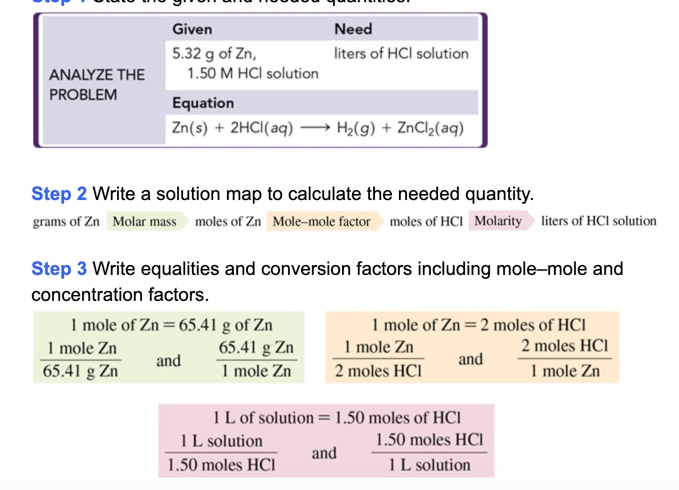 Source: chegg.com
Source: chegg.com
365 grams of HCl is dissolved in 162 grams of water. What is the mole equation. This means that your sample will contain. What is the excess reactant. Check all that apply.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in 1 mole of hcl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.