Your How many grams are in 1 mole of h2o2 images are ready in this website. How many grams are in 1 mole of h2o2 are a topic that is being searched for and liked by netizens today. You can Get the How many grams are in 1 mole of h2o2 files here. Find and Download all free images.
If you’re looking for how many grams are in 1 mole of h2o2 pictures information related to the how many grams are in 1 mole of h2o2 interest, you have come to the ideal site. Our site frequently provides you with hints for seeing the maximum quality video and image content, please kindly search and find more informative video articles and graphics that match your interests.
How Many Grams Are In 1 Mole Of H2o2. To add 10 mmole of H202 to your reaction from. First we need to calculate the molar mass molecular weight of H 2 O 2 hydrogen peroxide so we know how many grams equal one mole of the molecule. The molecular formula for Hydrogen Peroxide is H2O2. We are given 2 sig figs.
 From pinterest.com
From pinterest.com
Which and how many atoms does hydrogen peroxide H2O2 consist of. To add 10 mmole of H202 to your reaction from. So hydrogen peroxide has a molecular weight of 340146 grams per mole. The molecular formula for Hydrogen Peroxide is H2O2. Of moles 05 moles. Molar Mass of H₂O₂ - 2101 21600 3402 gmol.
This is approximately 000404 mole of hydrogen peroxide.
Technically there is no such thing but we will play like we can make 100 stuff. How many grams of hydrogen peroxide H2O2 are needed to produce 50 moles of water. Two hydrogen atoms can also bond with two oxygen O atoms peroxide making the formula H2O2. One mole of hydrogen peroxide H2O2 would consist of how many molecules. Cliffffy4h and 3 more users found this answer helpful. Molecular weight of Hydrogen Peroxide or grams.

From the balanced equation 4NH3 7O2 - 4NO2 6H2O. Molecular weight of Hydrogen Peroxide or grams. Note that rounding errors. How many grams of h2o2 are there in 1 mol of h2o2. 1 mole of peroxide H2O2 What is the molar mass of calcium chloride dihydrate CaCl2 2H2O.
 Source: youtube.com
Source: youtube.com
Hydrogen peroxide weighs 14425 gram per cubic centimeter or 1 4425 kilogram per cubic meter ie. The SI base unit for amount of substance is the mole. 1 mole of peroxide H2O2 What is the molar mass of calcium chloride dihydrate CaCl2 2H2O. Answer 1 of 2. The SI base unit for amount of substance is the mole.

Determine how many grams there are in the sample 8. Grendeldekt and 26 more users found this answer helpful. I assume you are talking about 100 H2O2. For H2O2 it is roughly 111616 34 dependent on t. 1 mole of peroxide H2O2 What is the molar mass of calcium chloride dihydrate CaCl2 2H2O.
 Source: toppr.com
Source: toppr.com
I think its 11 grams or 22 grams but im not sure. Hydrogen has an atomic mass of 1008 gmol and oxygen has an atomic mass of 159993 gmol. Mass volume x density. Using this as the starting point for the calculations we can determine the amount of O2 produced. Density of hydrogen peroxide is equal to 1 4425 kgm³.

This means the number of moles hydrogen peroxide is twice the number of moles of oxygen. Be sure to include the mass of all elements in the formula including the elements in. 1 1 161634 gmol. We are given 2 sig figs. This is approximately 000404 mole of hydrogen peroxide.
 Source: researchgate.net
Source: researchgate.net
We are given the amount of the peroxide that decomposes. An Introduction to Physical Science 14th Edition Edit edition Solutions for Chapter 13 Problem 11MC. Around 294 moles For this you need to use the equation. I think its 11 grams or 22 grams but im not sure. The molecular formula for Hydrogen Peroxide is H2O2.
 Source: pinterest.com
Source: pinterest.com
How many atoms of hydrogen are in a mole of hydrogen peroxide. MW H2O2 34 gmol. Be sure to include the mass of all elements in the formula including the elements in. This is approximately 000404 mole of hydrogen peroxide. To solve this first find the molar mass of H2O2 hydrogen peroxide by adding up the atomic masses of two H atoms and two O atoms found in the periodic table.

Grendeldekt and 26 more users found this answer helpful. Two hydrogen atoms can also bond with two oxygen O atoms peroxide making the formula H2O2. I assume you are talking about 100 H2O2. Molar Mass of O - 1600 gmol. Molecular weight of Hydrogen Peroxide or grams.
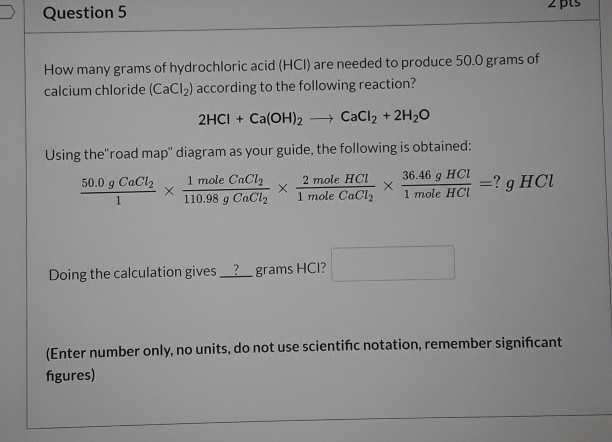 Source: chegg.com
Source: chegg.com
143 mol H2O2 1 mol O2 2 mol H2O2 715 mol O2 produced. The SI base unit for amount of substance is the mole. This means the number of moles hydrogen peroxide is twice the number of moles of oxygen. Mols H2O2 in 5075 g 50753401 149 mols. The molecular formula for Hydrogen Peroxide is H2O2.

We are given the amount of the peroxide that decomposes. Molar Mass of H₂O₂ - 2101 21600 3402 gmol. 1 mole of peroxide H2O2 What is the molar mass of calcium chloride dihydrate CaCl2 2H2O. An Introduction to Physical Science 14th Edition Edit edition Solutions for Chapter 13 Problem 11MC. We do as follows.
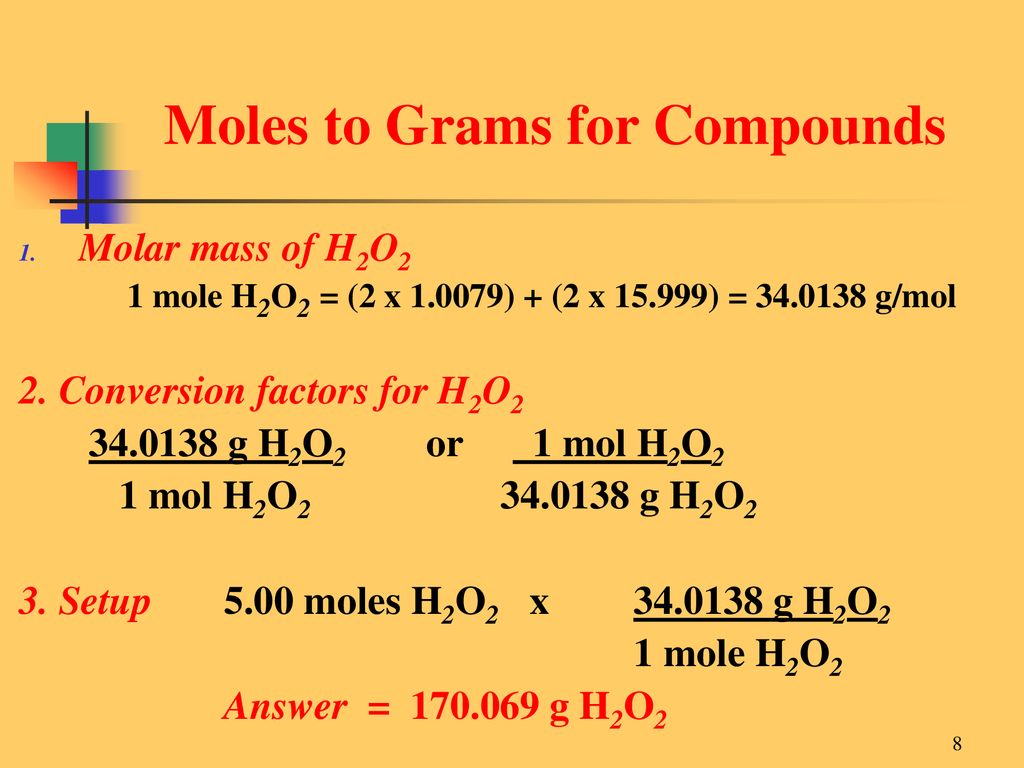 Source: slideplayer.com
Source: slideplayer.com
From the balanced equation 4NH3 7O2 - 4NO2 6H2O. Moles H 2 100 g H 2 x 1 mole 495 mol H 2 available 202 g Moles O 2 750 g O 2x 1 mole 234 mol O 2 available 320 g Method 1 Limiting Reactants Chapter 4 moles O 2 needed 495 mol H 2 x 1 mol O 2 2 mol H 2 248 moles O 2 Step 2 Pick H 2 and find the moles O 2 needed to react with all of the H 2 Limiting Reactants Chapter 4 Step 3. The molar mass of H2O2 is 3401 gmol. Density of hydrogen peroxide is equal to 1 4425 kgm³. The SI base unit for amount of substance is the mole.
 Source: doubtnut.com
Source: doubtnut.com
170 grams are given in the text of the problem. Molar Mass of H₂O₂ - 2101 21600 3402 gmol. Therefore the molarity of your 6 solution is 60 g34 gmol 1765 mol in 1 liter 1765 M. I assume you are talking about 100 H2O2. Mass 350 mL x 145 gmL 5075 g.
 Source: toppr.com
Source: toppr.com
I assume you are talking about 100 H2O2. 1 mole is equal to 1 moles Hydrogen Peroxide or 3401468 grams. The molar mass is calculated using the formula and the atomic weights on a periodic table. An Introduction to Physical Science 14th Edition Edit edition Solutions for Chapter 13 Problem 11MC. I bought some 90 stuff once and used it for some experiments.

An Introduction to Physical Science 14th Edition Edit edition Solutions for Chapter 13 Problem 11MC. Molar Mass of H₂O₂ - 2101 21600 3402 gmol. I bought some 90 stuff once and used it for some experiments. 1 mole is the same as 1 moles H2O2 or 3401468 grams. How many grams of CO2 are produced after complete reaction of.
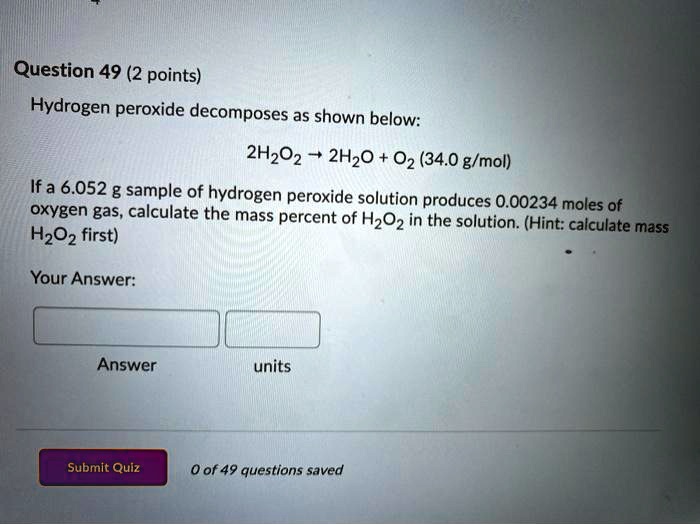 Source:
Source:
Hydrogen peroxide weighs 14425 gram per cubic centimeter or 1 4425 kilogram per cubic meter ie. From the balanced equation 4NH3 7O2 - 4NO2 6H2O. The molar mass of H2O2 is 3401 gmol. 2H2O2 2H2O O2. Cliffffy4h and 3 more users found this answer helpful.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in 1 mole of h2o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






