Your How many grams are in 1 mole of glucose images are available in this site. How many grams are in 1 mole of glucose are a topic that is being searched for and liked by netizens now. You can Get the How many grams are in 1 mole of glucose files here. Get all free vectors.
If you’re searching for how many grams are in 1 mole of glucose images information connected with to the how many grams are in 1 mole of glucose keyword, you have visit the ideal site. Our website frequently gives you suggestions for seeing the highest quality video and picture content, please kindly surf and locate more enlightening video articles and images that match your interests.
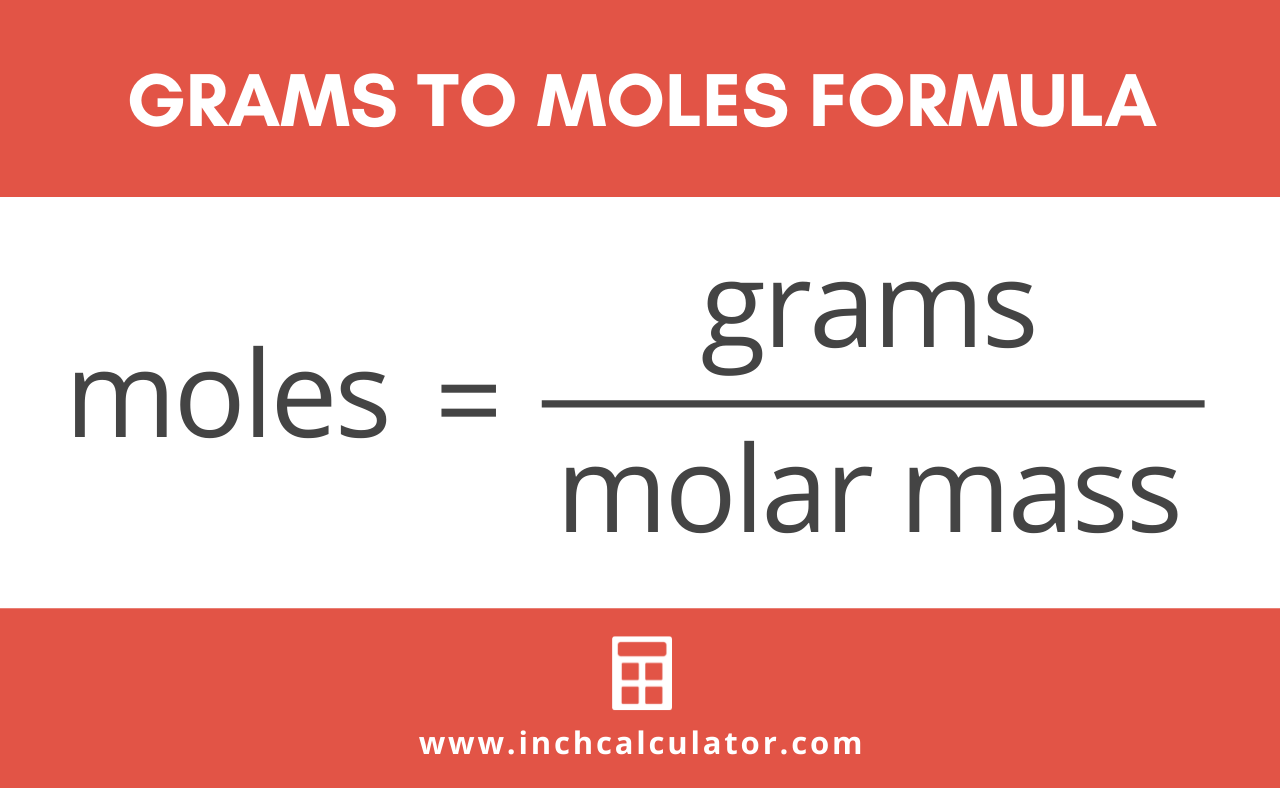
How Many Grams Are In 1 Mole Of Glucose. Now we can calculate the number of moles in given mass of glucose using the below formula Using the Formula numberofmolesgivenmassmolarmass we get. Glucose has a molar mass of 180156 gmol which means that one mole. The SI base unit for amount of substance is the mole. So one mol of sucrose is 3413 grams and would occupy about 215 ml volume.
 Grams To Moles Calculator Inch Calculator From inchcalculator.com
Grams To Moles Calculator Inch Calculator From inchcalculator.com
Solute per liter of solution. In one molecule of glucose there are 12 hydrogen 6 carbon and 6 oxygen atoms. 2 3200g 1 mol PbO2 2392g 1 mol O2. From the calculation we know that when one mole or 180 grams of glucose burns it releases 4950 kJ of energy the heat of reaction is -4950 kJmole. 1 mole is equal to 1 moles Glucose or 18015588 grams. The SI base unit for amount of substance is the mole.
What is a mole.
Click to explore further. 1 mole is equal to 1 moles C6H12O6 or 18015588 grams. 1 mol C 6 H 12 O 6 1802g 1 mol CO 2 4401g 1 mol C 6 H 12 O 6. Solute per liter of solution. The SI base unit for amount of substance is the mole. Molar mass of O 12 g mol-1.
 Source: youtube.com
Source: youtube.com
The SI base unit for amount of substance is the mole. Of moles Given or required weight Molecular weight Cheers m. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon. Furthermore if you dissolve 1 mole of a substance in enough water to make 1 liter L of solution you have made a. Molarity Moles of solute Volume of solution in litres 2 M n 1 L.
 Source: youtube.com
Source: youtube.com
How many moles will be there in 80gm of glucose. The SI base unit for amount of substance is the mole. Also Know how many moles are in one sugar. Molar mass of glucose is 180 gmol. 2 mol PbO2 Solution.
 Source: pinterest.com
Source: pinterest.com
The answer is rounded to three sig figs the number of sig figs you have for the number of moles of glucose. From the molecular formula C 6 H 12 O 6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. This would occupy about 117 ml of volume. Molecular weight of Glucose or mol The molecular formula for Glucose is C6H12O6. 100 mg x 1 mole180 grams x 1 gram1000 mg x 1 liter05 moles x 1000 mlliter 111 ml If you were to dilute 100 ml of the 05M glucose solution with 400 ml water what would be the concentration of the diluted solution.
 Source: youtube.com
Source: youtube.com
So one mol of sucrose is 3413 grams and would occupy about 215 ml volume. 10079 12120107 6159994 6 18016 gmol. Thus 1 mole of glucose weighs 180 g. The answer is 18015588. The SI base unit for amount of substance is the mole.
 Source: in.pinterest.com
Source: in.pinterest.com
Molecular weight of Glucose or grams The. Molecular weight of Glucose or mol The molecular formula for Glucose is C6H12O6. The SI base unit for amount of substance is the mole. We need 2 moles of glucose to prepare 1 litre of 2M solution. Molecular weight of Glucose or grams.
 Source: in.pinterest.com
Source: in.pinterest.com
900 g. 1 mole is equal to 1 moles C6H12O6 or 18015588 grams. 2 mol PbO2 Solution. This would occupy about 117 ml of volume. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
Molecular weight of C6H12O6 or grams This compound is also known as Glucose or Fructose or Galactose. 1 grams Glucose is equal to 00055507486072617. Thus 1 mole of glucose weighs 180 g. Now we can calculate the number of moles in given mass of glucose using the below formula Using the Formula numberofmolesgivenmassmolarmass we get. Cross multiply to get.
 Source: youtube.com
Source: youtube.com
The SI base unit for amount of substance is the mole. The SI base unit for amount of substance is the mole. How many grams Glucose in 1 mol. 1 molecule of glucose contains 6 atoms of C 12 atoms of H and 6 atoms of O 1 mole of glucose contains 6 moles of C atoms 12 moles of H atoms and 6 moles of O atoms. Of moles Given or required weight Molecular weight Cheers m.
 Source: in.pinterest.com
Source: in.pinterest.com
The SI base unit for amount of substance is the mole. 900g 180g 1 Mole 5 Moles To formulate - No. Convert moles Glucose to grams - Conversion of Measurement Units More information from the unit converter How many moles Glucose in 1 gramsThe answer is 00055507486072617We assume you are converting between moles Glucose and gramYou can view more details on each measurement unit. 1 mole is equal to 1 moles C6H12O6 or 18015588 grams. Molecular weight of Glucose or mol The molecular formula for Glucose is C6H12O6.
 Source: inchcalculator.com
Source: inchcalculator.com
The SI base unit for amount of substance is the mole. You can view more details on each measurement unit. 180g 900g 1 Mole. In this regard how much does a mole of glucose weigh. What is a mole.
 Source: youtube.com
Source: youtube.com
Molecular weight of Glucose or mol The molecular formula for Glucose is C6H12O6. Of moles Given or required weight Molecular weight Cheers m. Use coefficients of A and B from. Solute per liter of solution. Grams of solute per 100 ml of solvent.
 Source: youtube.com
Source: youtube.com
From the molecular formula C 6 H 12 O 6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. What is a mole. Molecular weight of C6H12O6 or grams This compound is also known as Glucose or Fructose or Galactose. So if one mole of glucose contains 6022 1023 molecules of glucose it follows that 400 moles of glucose will contain. Cross multiply to get.
 Source: study.com
Source: study.com
Molecular weight of Glucose or mol The molecular formula for Glucose is C6H12O6. 900 g. How many grams Glucose in 1 mol. 2mole 180 g mole 360 g. One molar solution of sucrose molecular mass 342 and a one molar solution of glucose molecular mass 180 will have exactly the same number of molecules per liter of solution even though the molecules are different in size.
 Source: no.pinterest.com
Source: no.pinterest.com
180g 1 Mole. The SI base unit for amount of substance is the mole. Click to explore further. Mass of two moles of Hydrogen atoms 2x 1 gmol 2 gmol. Cross multiply to get.
 Source: youtube.com
Source: youtube.com
Therefore Molar mass of C 6 H 12 O 6 6x12 12x1 6x16 72 12 96 180 g mol-1. Molar mass of O 12 g mol-1. This simply means that one Mole of glucose that is 60231023 molecules of glucose would weigh 180g. We need 2 moles of glucose to prepare 1 litre of 2M solution. From the calculation we know that when one mole or 180 grams of glucose burns it releases 4950 kJ of energy the heat of reaction is -4950 kJmole.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams are in 1 mole of glucose by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.