Your How many grams are in 1 mole of carbon images are ready in this website. How many grams are in 1 mole of carbon are a topic that is being searched for and liked by netizens now. You can Download the How many grams are in 1 mole of carbon files here. Find and Download all free vectors.
If you’re looking for how many grams are in 1 mole of carbon images information linked to the how many grams are in 1 mole of carbon topic, you have come to the right blog. Our website always gives you hints for viewing the highest quality video and image content, please kindly hunt and find more informative video content and graphics that match your interests.
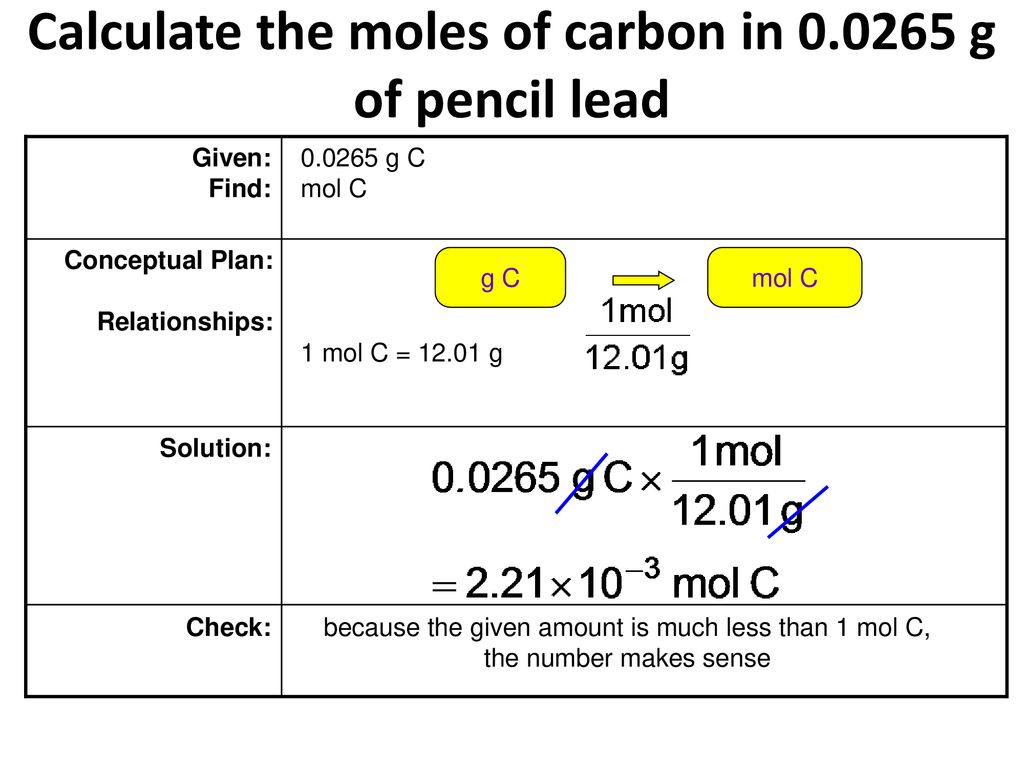
How Many Grams Are In 1 Mole Of Carbon. To find the mass of the C you multiply the moles of C by the mass of 1 mole of C atoms from the Periodic TAble. This answer is rounded to nearest one decimal place. Note that rounding errors may occur so always check the results. So the amount of carbon present in.
 1 6 Formula Mass And The Mole Concept Chemistry Libretexts From chem.libretexts.org
1 6 Formula Mass And The Mole Concept Chemistry Libretexts From chem.libretexts.org
Environment Canada publishes factors to estimate CO2. M m n m n M M m n m n M. Use dimensional analysis to calculate number of atoms. 1 mole of carbon. Use this page to learn how to convert between moles Carbon and gram. Overall there are 7845 grams in 1783 moles of CO2.
Which gives more CO2 per gram fuel.
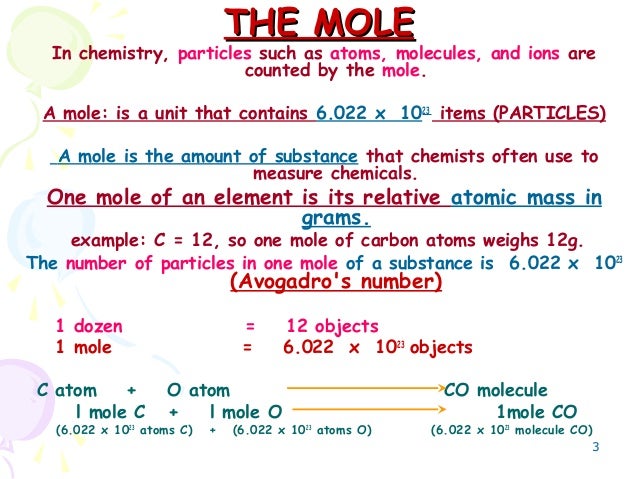
Type in your own numbers in the form to convert the units. Type in your own numbers in the form to convert the units. Calculate the number of atoms in 245 mol of copper. The molar mass of any compound is the mass in grams of one mole of that compound. 1 grams Carbon is equal to 0083259093974539 mole. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023.
 Source: chem.libretexts.org
Source: chem.libretexts.org
262 moles of C x 12 gmol 314 g of C in the 44 g sample. The mass of CO2 is. 1 mole is equal to 1 moles Carbon or 120107 grams. Learn this topic by watching Mole Concept Concept Videos. 1 grams Carbon is equal to 0083259093974539 mole.

How many kg are in in. Use this page to learn how to convert between moles Carbon Dioxide and gram. 1 grams Carbon Dioxide is equal to 0022722366761722 mole. This number is known as Avogadros number. Note that rounding errors may occur so always check the results.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
How many kg are in in. Grams of C 00987mole x 12gmole 118 grams. How many kg are in in. Use dimensional analysis to calculate number of atoms. One mole contains 60221023 entities.
 Source: geslab.net
Source: geslab.net
1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. Exactly 12 grams of pure carbon-12 powder is known as one mole. The molecular weights of carbon and oxygen are 12 and 16. 1 grams of carbon is equal to 0083259093974539 mole. Environment Canada publishes factors to estimate CO2.
 Source: cdli.ca
Source: cdli.ca
M m n m n M M m n m n M. 262 moles of C x 12 gmol 314 g of C in the 44 g sample. ONE MOL OF CO2 has 6021023 molecules of CO2. 1 grams Carbon Dioxide is equal to 0022722366761722 mole. There are 121024 atoms in 24 grams of carbon.
 Source: youtube.com
Source: youtube.com
ONE MOL OF CO2 has 6021023 molecules of CO2. 1 mole is equal to 1 moles Carbon Dioxide or 440095 grams. 440 1783 7845 grams. 1 grams of carbon is equal to 0083259093974539 mole. From the above concept of mole we tell that one mole of carbon contains.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. The SI base unit for amount of substance is the mole. This number is known as Avogadros number. To find the mass of the C you multiply the moles of C by the mass of 1 mole of C atoms from the Periodic TAble. This answer is rounded to nearest one decimal place.
 Source: slideplayer.com
Source: slideplayer.com
Type in your own numbers in the form to convert the units. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23. So the amount of carbon present in. The molar mass of any compound is the mass in grams of one mole of that compound. 1 mole is equal to 1 moles Carbon Dioxide or 440095 grams.
 Source: geslab.net
Source: geslab.net
The molecular weight of one carbon and two oxygen atoms s is 2 x 16 12 44 rounded off 01 moles of CO2 01 x 44 44 grams. 1 mole is equal to 1 moles Carbon or 120107 grams. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23. 2 mol C 60221023 atomsmol 121024 atoms C to two significant figures. Not only is there a numerical relationship but there is also a mass relationship.
 Source: clutchprep.com
Source: clutchprep.com
1 mole is equal to 1 moles CO2 or 440095 grams. To find the mass of the C you multiply the moles of C by the mass of 1 mole of C atoms from the Periodic TAble. How many atoms are found in 12 grams 1 mole of carbon 12. This answer is rounded to nearest one decimal place. Learn this topic by watching Mole Concept Concept Videos.
 Source: geslab.net
Source: geslab.net
How many atoms are found in 12 grams 1 mole of carbon 12. Note that rounding errors may occur so always check the results. 440 1783 7845 grams. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 1023. 1 grams of carbon is equal to 0083259093974539 mole.
 Source: slideplayer.com
Source: slideplayer.com
Not only is there a numerical relationship but there is also a mass relationship. How many GRAMS of carbon tetrabromide can be produced from 105 grams of bromine. This number is known as Avogadros number. Type in your own numbers in the form to convert the units. Exactly 12 grams of pure carbon-12 powder is known as one mole.
 Source: slideshare.net
Source: slideshare.net
Type in your own numbers in the form to convert the units. How many kg are in in. ONE MOL OF CO2 has 6021023 molecules of CO2. When you are talking about 1 mole of sucrose its the same as saying 1 mole of sucrose atoms so there are Avogadros number of atoms in one mole of sucrose or carbon or anything measured in moles. Not only is there a numerical relationship but there is also a mass relationship.
 Source: youtube.com
Source: youtube.com
Type in your own numbers in the form to convert the units. 1 120 2 160 440 gmol. Substitute the known values and simplify. Use dimensional analysis to calculate number of atoms. One mole of carbon dioxide molecules has a mass of 4401g while one mole of sodium sulfide formula units has a mass of 7804g.
 Source: youtube.com
Source: youtube.com
1 grams Carbon Dioxide is equal to 0022722366761722 mole. Avogadros number And given the molar weights of carbon and oxygen we know that each mol of CO2 weighs 121616 grams or 4401 grams when accounting for isotopes. Type in your own numbers in the form to convert the units. How many atoms are found in 12 grams 1 mole of carbon 12. How many moles are in a gram of sugar.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in 1 mole of carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






