Your How many grams are in 1 mole of calcium ca images are ready in this website. How many grams are in 1 mole of calcium ca are a topic that is being searched for and liked by netizens now. You can Get the How many grams are in 1 mole of calcium ca files here. Find and Download all free photos and vectors.
If you’re looking for how many grams are in 1 mole of calcium ca images information connected with to the how many grams are in 1 mole of calcium ca keyword, you have pay a visit to the right site. Our website frequently gives you suggestions for seeking the highest quality video and image content, please kindly surf and find more enlightening video content and graphics that match your interests.
How Many Grams Are In 1 Mole Of Calcium Ca. Grams cancel and mol-1 move to top to become moles 626g4008gmol-1 156 moles Ca Something went wrong. 1 mole is equal to 1 moles CH4 or 1604246 grams. Moles 2000 169873 1177 moles. The SI base unit for amount of substance is the mole.
 How Many Moles Are 5 Grams Of Calcium Atomic Mass Of Calcium 40 U Youtube From youtube.com
How Many Moles Are 5 Grams Of Calcium Atomic Mass Of Calcium 40 U Youtube From youtube.com
3910 grams is the molar mass of one mole of K. 35molCa 40078 g mol 140 g Ca to two significant figures. How many grams is in 1000 moles of calcium Ca. Number of moles Gram weight Atomic weight of substance. Calcium Ca Molar Mass. The SI base unit for amount of substance is the mole.
Note that rounding errors may occur so always check the results.
This is not the eq. There is one atom of calcium which has a molar mass of 40078 gmol. As CaOH2 has two OH per molecule or 2x the moles. 3910 grams is the molar mass of one mole of K. Type in your own numbers in the form to convert the units. Use the molecular formula to find the molar mass.
 Source: toppr.com
Source: toppr.com
There is one atom of calcium which has a molar mass of 40078 gmol. We know that Atomic weight of calcium Ca is 40078 u. How many grams is in 1000 moles of calcium Ca. The molar mass of calcium is 40078 gmol. To obtain the number of moles divide the mass of compound by the molar mass of the compound expressed in grams.
 Source: slideplayer.com
Source: slideplayer.com
Of oxygen atoms 15 60221023 90331023 atoms Mass of Oxygen atoms 15 16 24 g. Type in your own numbers in the form to convert the units. Of oxygen atoms 15 60221023 90331023 atoms Mass of Oxygen atoms 15 16 24 g. There are about 3 significant digits because 102 has the LEAST figures available. Molar mass is the mass in grams in one mole of a substance.

Note that rounding errors may occur so always check the results. Note that rounding errors may occur so always check the results. Might 07 2020 Reply and Rationalization. 1 grams Calcium is equal to 0024951344877489 mole. I 60 g of calcium.
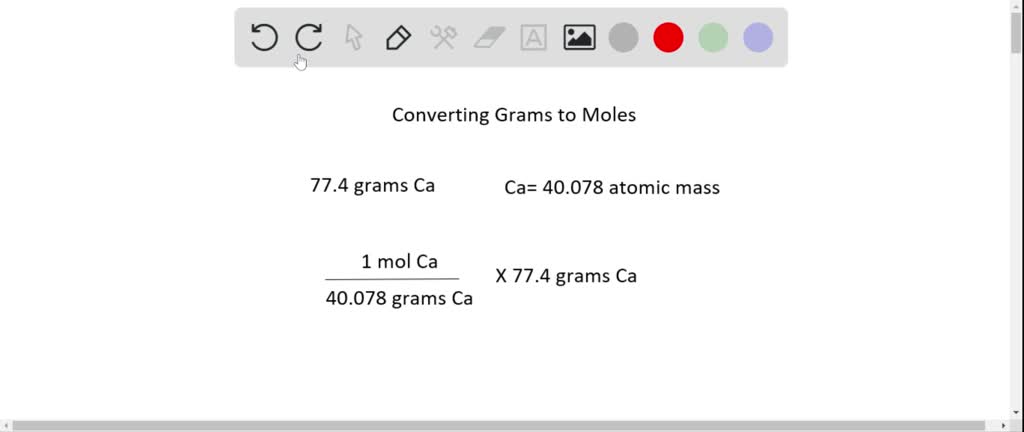 Source: numerade.com
Source: numerade.com
There are about 3 significant digits because 102 has the LEAST figures available. This fraction can be multiplied by the number of moles which is 4. Might 07 2020 Reply and Rationalization. The number of atoms can also be calculated using Avogadros Constant 60221417910 23 one mole of substance. 1 mole is equal to 1 moles CH4 or 1604246 grams.
 Source: numerade.com
Source: numerade.com
Grams 58443 5 292215 g Molar mass of AgNO3 is 169873 2 kg AgNO3 is equal to how many moles. Given that Ca 40 u. We know that Atomic weight of calcium Ca is 40078 u. This is not the eq. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass.
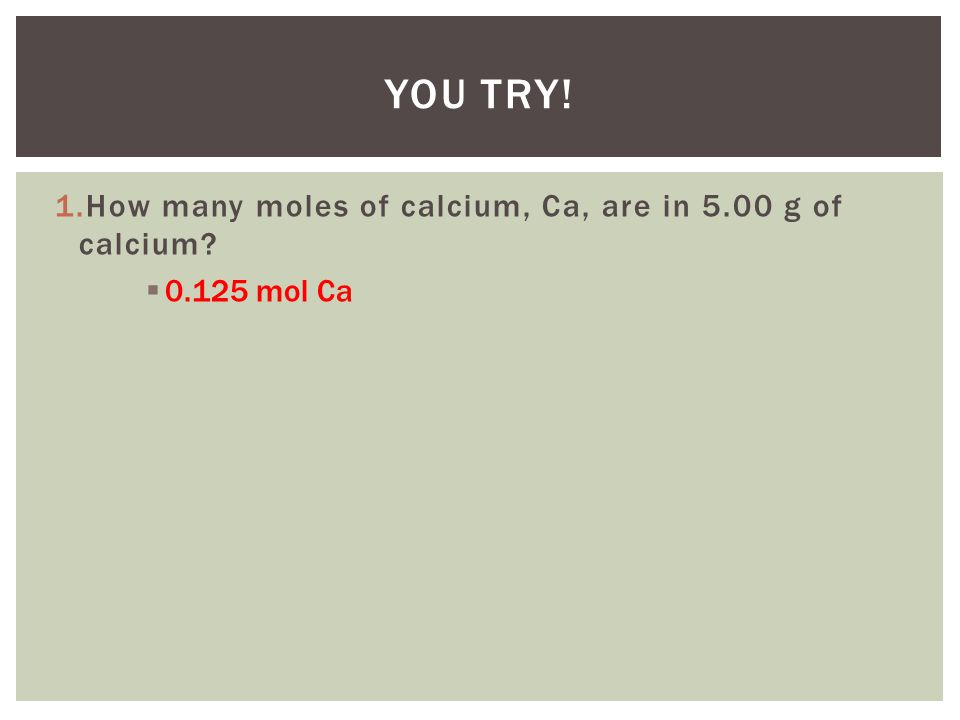 Source: slideplayer.com
Source: slideplayer.com
The molar mass of calcium nitrate is 1640878 gmol. 401 g Ca 1 mol Ca 22 g Ca. As CaOH2 has two OH per molecule or 2x the moles. Grams cancel and mol-1 move to top to become moles 626g4008gmol-1 156 moles Ca Something went wrong. What number of grams are in 1 mole of calcium ca.

How do I calculate moles. 1 grams Calcium is equal to 0024951344877489 mole. Mole of CaCO3 50g100g 05 05 mole of CaCO3 contains 15 moles of oxygen atoms No. Number of moles Gram weight Atomic weight of substance. Type in your own numbers in the form to convert the units.
 Source: youtube.com
Source: youtube.com
Number of moles Gram weight Atomic weight of substance. Moles 2000 169873 1177 moles. Molar mass is the mass in grams in one mole of a substance. So the mole-to-mole ratio of calcium oxide and water is 11. There are about 3 significant digits because 102 has the LEAST figures available.
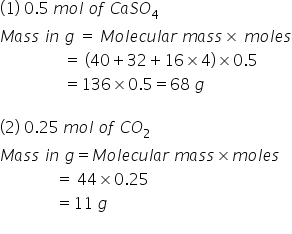 Source: topperlearning.com
Source: topperlearning.com
Molar mass is the mass in grams in one mole of a substance. How many grams are contained in 054 moles of calcium. G Ca 054 mol Ca. 401 g Ca 1 mol Ca 22 g Ca. The number of atoms can also be calculated using Avogadros Constant 60221417910 23 one mole of substance.
 Source: numerade.com
Source: numerade.com
The total number of atoms in a substance can also be determined by using the relationship between grams moles and atoms. Number of oxygen atoms. The number of atoms can also be calculated using Avogadros Constant 60221417910 23 one mole of substance. 1 mole is equal to 1 moles CH4 or 1604246 grams. 35molCa 40078 g mol 140 g Ca to two significant figures.
 Source: nagwa.com
Source: nagwa.com
Calcium Ca Molar Mass. The number of atoms can also be calculated using Avogadros Constant 60221417910 23 one mole of substance. 401 g Ca 1 mol Ca 22 g Ca. How many grams are contained in 054 moles of calcium. 1 mole of calcium fluoride has a mass of 400821899984 780797 grams if one mole has a mass of 780797 grams then 25 moles have.

The SI base unit for amount of substance is the mole. This is not the eq. As we know that one mole is equal to the one gram atom which is also equal to the molar mass. Note that rounding errors may occur so always check the results. Moles 2000 169873 1177 moles.
 Source: youtube.com
Source: youtube.com
102 grams x 1 mol of Ca 4008g 0254491018. Mole of CaCO3 50g100g 05 05 mole of CaCO3 contains 15 moles of oxygen atoms No. There are about 3 significant digits because 102 has the LEAST figures available. 1 mole is equal to 1 moles CH4 or 1604246 grams. The reply is 0024951344877489.
 Source: slideplayer.com
Source: slideplayer.com
3910 grams is the molar mass of one mole of K. The total number of atoms in a substance can also be determined by using the relationship between grams moles and atoms. Solution Molecular mass of CaCO3 4012316 100 No. Grams can be canceled leaving the moles of K. To obtain the number of moles divide the mass of compound by the molar mass of the compound expressed in grams.
 Source: slideplayer.com
Source: slideplayer.com
The reply is 0024951344877489. Calcium Ca Molar Mass. So the mole-to-mole ratio of calcium oxide and water is 11. Note that rounding errors may occur so always check the results. Type in your own numbers in the form to convert the units.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in 1 mole of calcium ca by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






