Your How many grams are in 1 mole of al2so43 images are ready. How many grams are in 1 mole of al2so43 are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams are in 1 mole of al2so43 files here. Find and Download all royalty-free photos and vectors.
If you’re looking for how many grams are in 1 mole of al2so43 images information connected with to the how many grams are in 1 mole of al2so43 topic, you have pay a visit to the ideal site. Our site frequently gives you suggestions for refferencing the maximum quality video and picture content, please kindly surf and find more enlightening video articles and images that fit your interests.
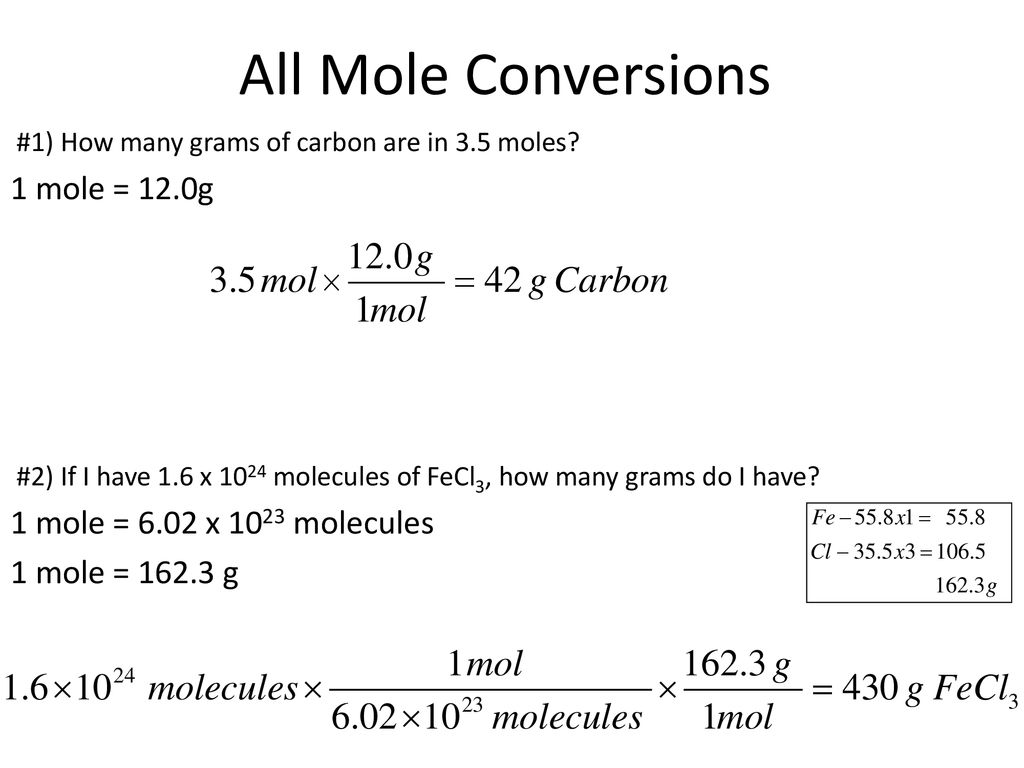
How Many Grams Are In 1 Mole Of Al2so43. 1 mole of K 2 S O4 Al2 S O4 3 24H 2 O contains 4 gram ions of S O42 125 mole of K 2 S O4 Al2 S O4 3 24H 2 O contains 4125 gram ions 5 gram ions of S O42. Molar mass of Al2 SO43. So 1 mol of Al2 SO43 yields 3 mols of SO4 -2 ions. Mass O 16 x 0096154 g.
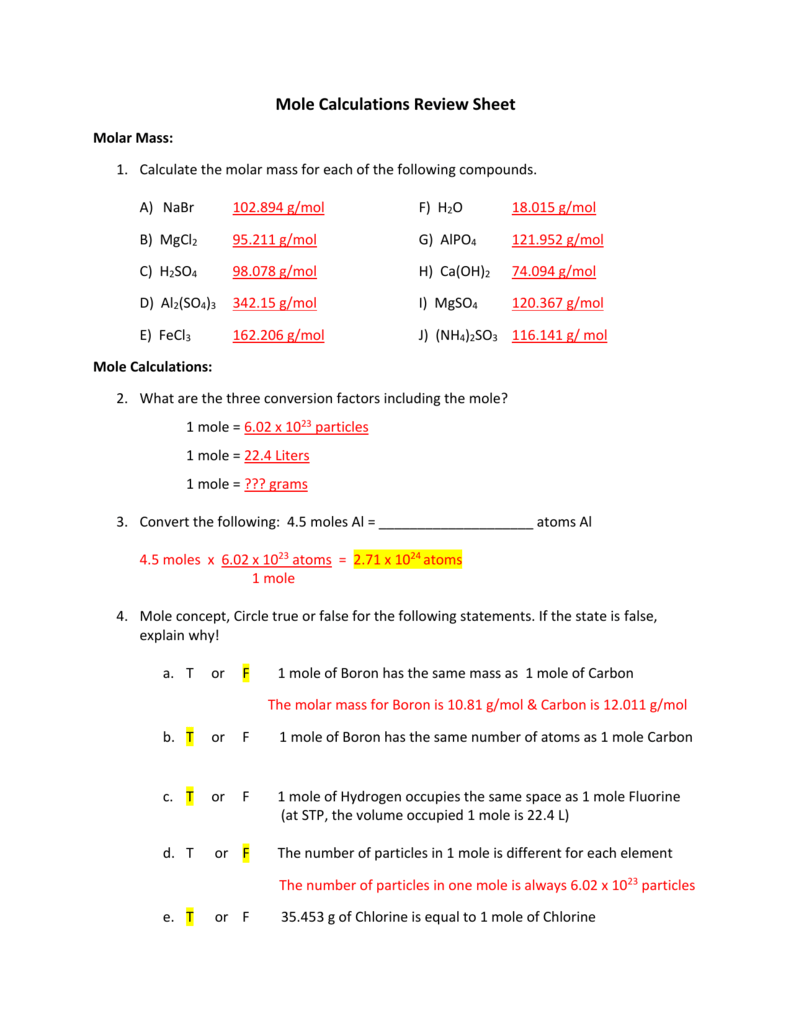 Mole Calculations Review Sheet From studylib.net
Mole Calculations Review Sheet From studylib.net
If you have 3 moles of Al2 SO43 you obviously have 9 moles atg of sulfur. First we have to calculate the number of moles of oxygen present in the compound. Get control of 2021. There are 110 10 24 O atoms in 476 g Al 2 CO 3 3. How many atoms is 580 moles. The SI base unit for amount of substance is the mole.
Moles O 12 x 000800096.
There are 110 10 24 O atoms in 476 g Al 2 CO 3 3. Then in a formula unit of Al2SO43 there are two aluminum ions and three sulfate ions. The number of molecules in a mole. How many grams of aluminum sulfate are produced from 267 mol sulfuric acid in the following unbalanced equation. Keeping this in view how many moles are there in 684 g of al2 so4 3. Convert between Al2 SO43 weight and moles.
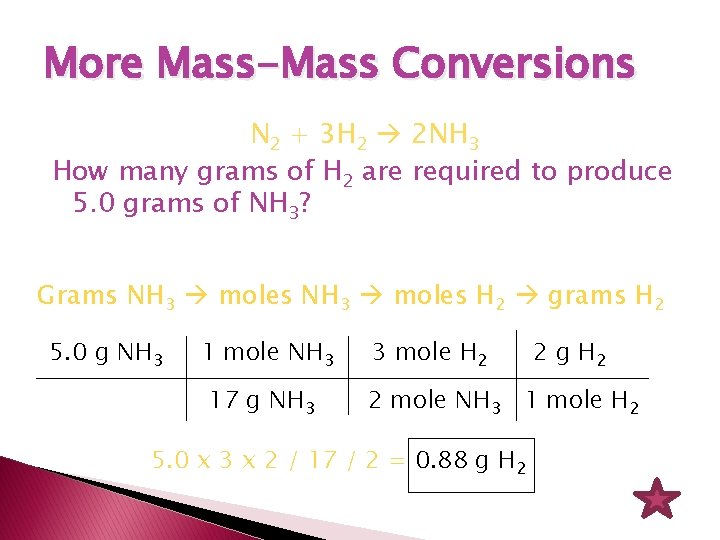 Source: slidetodoc.com
Source: slidetodoc.com
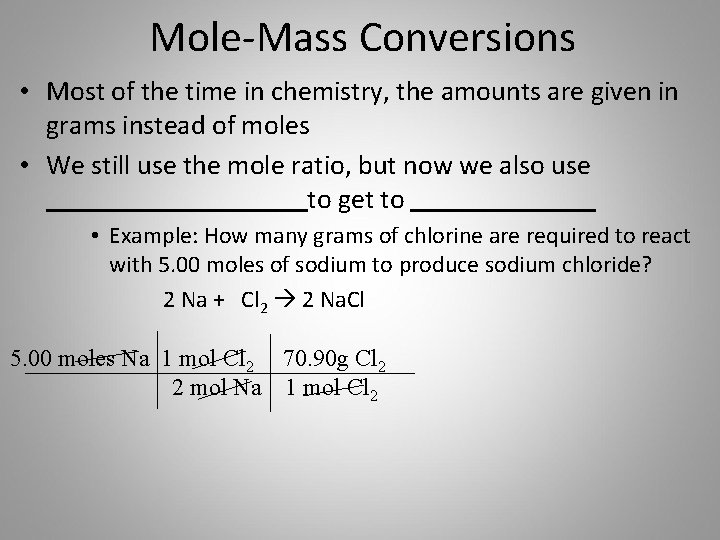
This compound is also known as Aluminium Sulfate. In the following reaction how many grams of Al2SO43 can be produced from 327 grams of aluminum. Or two aluminum three sulfur and twelve oxygen atoms. So in 125 moles of potash alum 4 125 moles of SO42- are present ie. Al203s H2SO4aq Al2SO43aq H2O1.
 Source: youtube.com
Source: youtube.com
1 grams Al2SO43 is equal to 00029226872416366 mole. One molecule of CCl4 has 1 carbon atom and 4 chlorine atoms. 580 mol He 60221023 atoms He 1 mol He. That is the molar mass of a problem is the fixed in grams every mole of 6022 1023 atoms molecules or formula systems of that substance. If you have 3 moles of Al2 SO43 you obviously have 9 moles atg of sulfur.
 Source: toppr.com
Source: toppr.com
So the molecular weight of CCl4 is 1201 43545 15381 gmol. Correct answer to the question For the reaction 2AI 3H2SO4 - Al2SO43 3H2 If 225 g of Al is added to 436g of H2SO4 calculate a How many grams of Al2SO43 would be formed. What is the percent composition of compound that contains 408 g carbon and 544 oxygen. 2Al 3H2SO4 arrow Al2SO43 3H2. Al2SO4 324H2O is commonly known as potash alum.
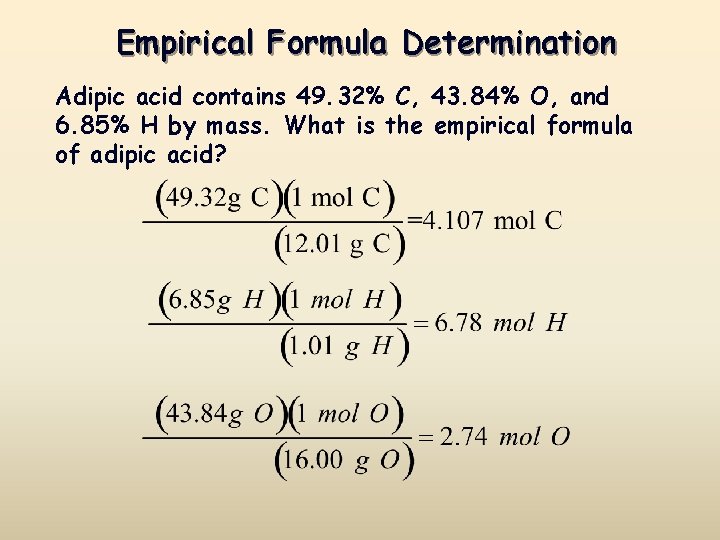 Source: slidetodoc.com
Source: slidetodoc.com
Keeping this in view how many moles are there in 684 g of al2 so4 3. If you have 3 moles of Al2 SO43 you obviously have 9 moles atg of sulfur. This compound is soluble in water. Its boiling point is 214 degrees Fahrenheit and the density is 271 grams per cubic centimeter. That is the molar mass of a problem is the fixed in grams every mole of 6022 1023 atoms molecules or formula systems of that substance.
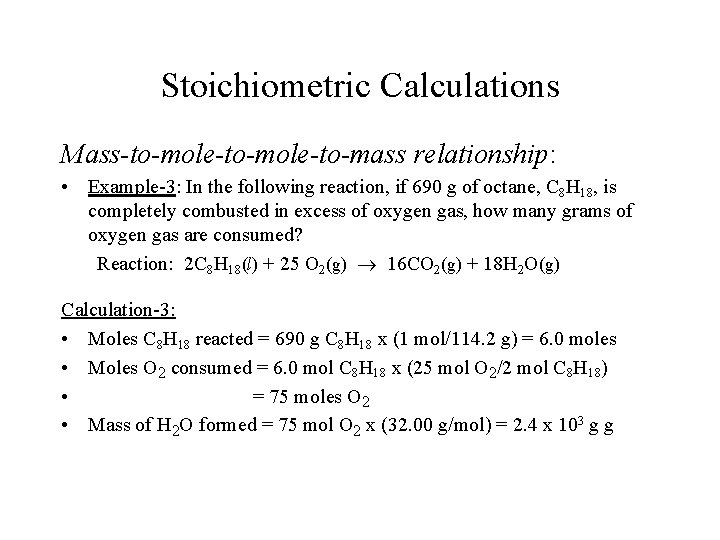 Source: slidetodoc.com
Source: slidetodoc.com
1 grams Al2SO43 is equal to 00029226872416366 mole. 6022 10 23 O atoms in 1 mole O. Al2SO4 324H2O is commonly known as potash alum. That is the molar mass of a problem is the fixed in grams every mole of 6022 1023 atoms molecules or formula systems of that substance. Avogadros number gives us the number of entities present in 1 mole of anything.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of Al2 SO43 or grams. First we have to calculate the number of moles of oxygen present in the compound. What is the percent composition of compound that contains 408 g carbon and 544 oxygen. This compound is also known as Aluminium Sulfate. Molecular weight of Al2 SO43 or grams.
 Source: slideplayer.com
Source: slideplayer.com
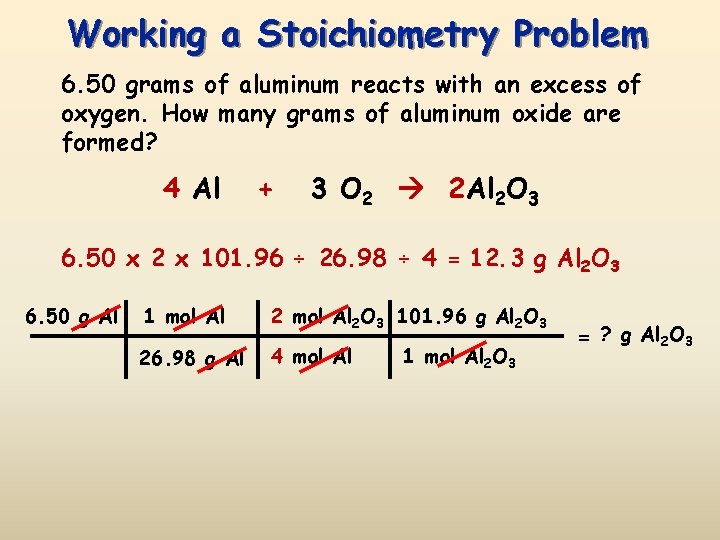
Mass of 1 mole of AL2SO43 340g. How many molecules room in a gram that a compound. In the following reaction how many grams of Al2SO43 can be produced from 327 grams of aluminum. By unitary method we get 340g1 mole. So 1 mol of Al2 SO43 yields 3 mols of SO4 -2 ions.
 Source: studylib.net
Source: studylib.net
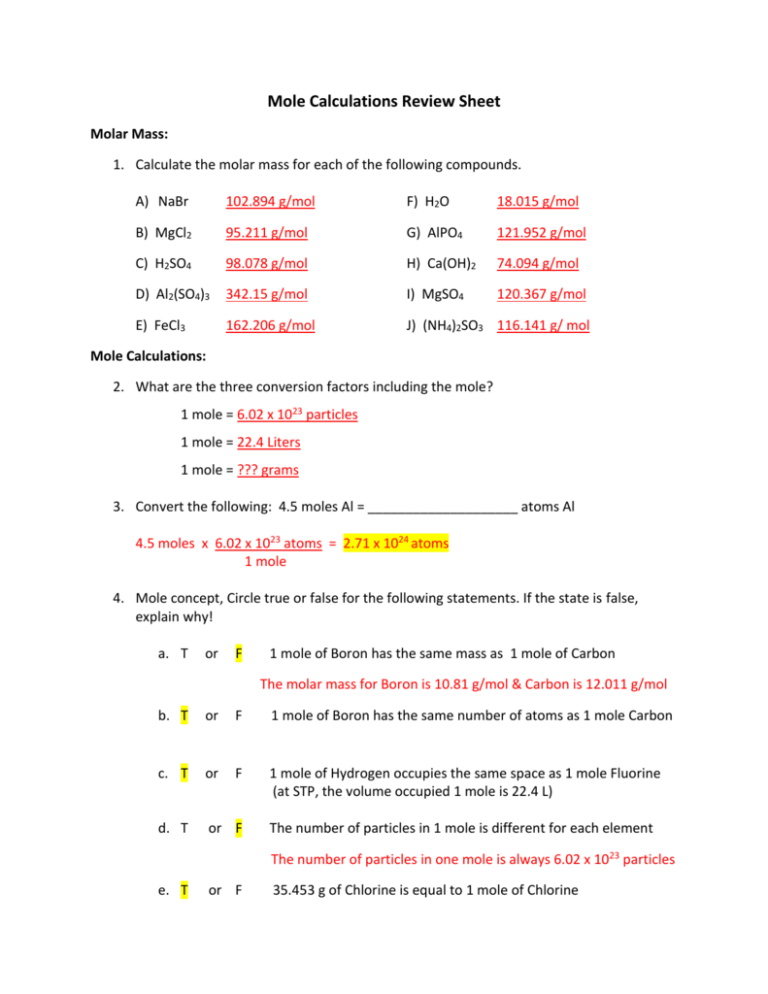
In one one complex molecule of potash alum 4 ions of SO42- are present. That is the molar mass of a problem is the fixed in grams every mole of 6022 1023 atoms molecules or formula systems of that substance. Subsequently question is what is the formula mass of al2 so4 3. Compound name is aluminium sulfate. 1 mole contains 60221023 atoms.
 Source: slidetodoc.com
Source: slidetodoc.com
34 Related question Answers Found How numerous grams is al2 so4 3. This compound is soluble in water. 34 Related question Answers Found How numerous grams is al2 so4 3. Or two aluminum three sulfur and twelve oxygen atoms. If you have 3 moles of Al2 SO43 you obviously have 9 moles atg of sulfur.
 Source: studylib.net
Source: studylib.net
1 mole contains 60221023 atoms. Al203s H2SO4aq Al2SO43aq H2O1. Use this page to learn how to convert between grams Al2 SO43 and mole. Mass of 1 mole of AL2SO43 340g. Mass O 16 x 0096154 g.

So 1 mol of Al2 SO43 yields 3 mols of SO4 -2 ions. Track your food intake exercise sleep and meditation for free. How many grams of aluminum sulfate are produced from 267 mol sulfuric acid in the following unbalanced equation. Al203s H2SO4aq Al2SO43aq H2O1. How many moles of Al2SO43 can form Chemistry According to the reaction 2Al3H2SO43H2Al2SO43 the total number of moles of H2SO4 needed to react completely with 50 mol of AL is 1.

One molecule of CCl4 has 1 carbon atom and 4 chlorine atoms. - The concentration of ammonia 17031 gmol can be. Molar mass of Al2 SO43. Its boiling point is 214 degrees Fahrenheit and the density is 271 grams per cubic centimeter. 342150876 grams How numerous atoms room in h2o.
 Source: slidetodoc.com
Source: slidetodoc.com
- The concentration of ammonia 17031 gmol can be. Molar mass of Al2 SO43. How many moles of Al2SO43 can form Chemistry According to the reaction 2Al3H2SO43H2Al2SO43 the total number of moles of H2SO4 needed to react completely with 50 mol of AL is 1. Avogadros number gives us the number of entities present in 1 mole of anything. Number of moles M o l e c u l a r m a s s M a s s Given mass of A l 2 S O 4 3 6 0 g Molecular mass of A l 2 S O 4 3 2 2 7 3 3 2 4 1 6 3 4 2 g.
 Source: studylib.net
Source: studylib.net
32 grams per mole x 9 moles 288 grams of sulfur. Molar mass of Al2 SO43. Moles O 12 x 000800096. 1 grams Al2SO43 is equal to 00029226872416366 mole. In the following reaction how many grams of Al2SO43 can be produced from 327 grams of aluminum.

The mass in grams of a mole of sulfur sulfurs molar mass can be found on a periodic table of the elements to be 32 grams per mole. Or two aluminum three sulfur and twelve oxygen atoms. 1 mole is equal to 1 moles Al2 SO43 or 342150876 grams. During the production of one mole of compound change in enthalpy takes places this change in entha. The stoichiometric ratio that will be used is 1 mole Al2SO43 12 moles O 1 m o l e.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams are in 1 mole of al2so43 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






