Your How many grams aluminum equal 35 moles aluminum images are ready in this website. How many grams aluminum equal 35 moles aluminum are a topic that is being searched for and liked by netizens today. You can Get the How many grams aluminum equal 35 moles aluminum files here. Find and Download all royalty-free photos and vectors.
If you’re searching for how many grams aluminum equal 35 moles aluminum images information related to the how many grams aluminum equal 35 moles aluminum topic, you have visit the right blog. Our website always gives you hints for seeking the maximum quality video and image content, please kindly hunt and find more informative video articles and images that fit your interests.
How Many Grams Aluminum Equal 35 Moles Aluminum. 35 Related Question Answers Found. Aluminum weighs 2699 gram per cubic centimeter or 2 699 kilogram per cubic meter ie. How many moles of barium are in a sample containing 425 x 1026 atoms of barium. 1 mole is equal to 1 moles KMnO4 or 158033949 grams.
 How Many Moles Of Al2 So4 3 Would Be In 50 G Of The Substance From toppr.com
How Many Moles Of Al2 So4 3 Would Be In 50 G Of The Substance From toppr.com
How many grams are in 1 mole of aluminum. Equal stoichiometric numbers of moles of acid and base are present in the flask and the pH should be. 2Al s 6H2O g 2Al OH3 s 3H2 g. The SI base unit for amount of substance is the mole. Density of aluminum is equal to 2 699 kgm³. Cl 1 x 3545 amu 35 45 amu 58 45 amu B Moles Mole is a chemist counting unit used to count atoms molecules or compounds by weighting.
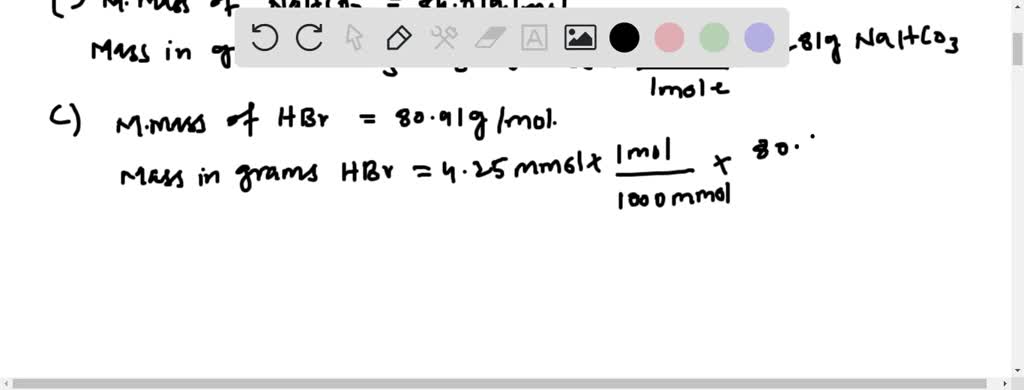
So here we can write another proportion to over four is equal to next over 523 and X is equal to my second.
You can view more details on each measurement unit. The SI base unit for amount of substance is the mole. Thus the total number of moles in 9. The SI base unit for amount of substance is the mole. 189 If 160 grams of aluminum oxide were actually produced what is the percent yield of the reaction below given that you start with 100 g of Al and 190 grams of O2. Molecular weight of Aluminium or mol The molecular formula for Aluminium is Al.
 Source: numerade.com
Source: numerade.com
How many grams are in 90 moles of aluminum. 625g of copper 1 mol 977 mol Cu 64 g Cu 2. Answer 1 mole 602 x 10 23 objects. You can view more details on each measurement unit. 1 mole is equal to 1 moles Aluminium or 26981538 grams.
 Source: in.pinterest.com
Source: in.pinterest.com
At 20C 68F or 29315K at standard atmospheric pressure. 3 mol NaOH 00616 mol FeCl 3 x 0185 mol NaOH 1 mol FeCl 3 C Moles to mass. The atomic masses of aluminum and oxygen are 270 grams and 160 grams respectively. 35 Related Question Answers Found. A Mass to moles.
 Source: youtube.com
Source: youtube.com
35 Related Question Answers Found. 1 grams Aluminum is equal to 0. Assume 22 grams of aluminum sulfide Al2S3 reacts with water H2O to produce aluminum hydroxide AlOH3 and hydrogen sulfide H2S. This means that if you know how many moles of aluminium you have in 455 g you can use Avogadros number to. M 356 g m 356 g.
 Source: sciencedirect.com
Source: sciencedirect.com
How many grams of solid aluminum sulfide can be prepared by the reaction of. Note that rounding errors may occur so always check the results. The atomic mass on the periodic table is the number of grams associated with one mole of that element. Note that rounding errors may occur so always check the results. 16849307 pounds lbs of Aluminum fit into 1 cubic foot.
 Source: mdpi.com
Source: mdpi.com
The given sample mass is. The atomic masses of aluminum and oxygen are 270 grams and 160 grams respectively. 189 If 160 grams of aluminum oxide were actually produced what is the percent yield of the reaction below given that you start with 100 g of Al and 190 grams of O2. Dividing 269860210 23 yields a mass of 44810 23 g for one aluminum atom. We assume you are converting between grams Aluminum and mole.
 Source: mdpi.com
Source: mdpi.com
A Mass to moles. Assume 22 grams of aluminum sulfide Al2S3 reacts with water H2O to produce aluminum hydroxide AlOH3 and hydrogen sulfide H2S. You can view more details on each measurement unit. Dividing 269860210 23 yields a mass of 44810 23 g for one aluminum atom. 1 mole is equal to 1 moles Aluminium or 26981538 grams.
 Source: mdpi.com
Source: mdpi.com
How many grams Aluminium in 1 mol. How many moles of Al4C3 were reacted when 355 x 10 35 formulas units of aluminum hydroxide were produced. How many grams of water are needed to react with 1000 moles of Al4C3. Aluminum oxide al2o3 is used as a filler for paints and varnishes as well as in the manufacture of electrical insulators. Aluminum weighs 2699 gram per cubic centimeter or 2 699 kilogram per cubic meter ie.
 Source: toppr.com
Source: toppr.com
A student reacts 34 moles of aluminum with excess water according to the following equation. How many grams are in 1 mole of aluminum. MM2698 gmol M M 2698 g. 625g of copper 1 mol 977 mol Cu 64 g Cu 2. Aluminum weighs 2699 gram per cubic centimeter or 2 699 kilogram per cubic meter ie.

The answer is 26981538. This means that the mass of one mole of aluminium atoms will be 26982 g. The problem requires that you determine the mass of water H2O needed for the reaction to occur. Equal stoichiometric numbers of moles of acid and base are present in the flask and the pH should be. How many grams Aluminum in 1 mol.
 Source: studylib.net
Source: studylib.net
How many grams of water are needed to react with 1000 moles of Al4C3. How many moles of barium are in a sample containing 425 x 1026 atoms of barium. We assume you are converting between grams Aluminium and mole. The SI base unit for amount of substance is the mole. How many moles of H ions are present in 750ml of 065 M hydrochloric acid.
 Source:
Source:
How many grams of aluminum are required to produce 870 moles of aluminum chloride. Equal stoichiometric numbers of moles of acid and base are present in the flask and the pH should be. How many moles of Al4C3 were reacted when 355 x 10 35 formulas units of aluminum hydroxide were produced. Molecular weight of Aluminum or mol The molecular formula for Aluminum is Al. 1 mole is equal to 1 moles Aluminum or 26981538 grams.
 Source: slideshare.net
Source: slideshare.net
1 mole is equal to 1 moles KMnO4 or 158033949 grams. Density of aluminum is equal to 2 699 kgm³. Cl 1 x 3545 amu 35 45 amu 58 45 amu B Moles Mole is a chemist counting unit used to count atoms molecules or compounds by weighting. Use this page to learn how to convert between moles Aluminium Hydroxide and gram. How many grams Aluminium in 1 mol.
 Source: mdpi.com
Source: mdpi.com
How many grams of solid aluminum sulfide can be prepared by the reaction of. Dividing 269860210 23 yields a mass of 44810 23 g for one aluminum atom. Okay so theres a 4 to 2 ratio here and we want to see that 5235 523 Moles of aluminum are forced to react. 425 x 1026 atoms of barium 1 mol 706 mol 602 x 1023 atoms 3. How many moles of H ions are present in 750ml of 065 M hydrochloric acid.
 Source: khanacademy.org
Source: khanacademy.org
12mol H2O 1mol Al4C3 X 1801g H2O 1mol H2O 2160 x 104g H2O. How many moles are in 35 grams of aluminum. The atomic mass on the periodic table is the number of grams associated with one mole of that element. How many grams are contained in a 0893 mol sample of methane CH4. Note that rounding errors may occur so always check the results.
 Source: toppr.com
Source: toppr.com
The SI base unit for amount of substance is the mole. Use this page to learn. How many moles are in 35 grams of aluminum. Okay so theres a 4 to 2 ratio here and we want to see that 5235 523 Moles of aluminum are forced to react. At 20C 68F or 29315K at standard atmospheric pressure.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams aluminum equal 35 moles aluminum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





