Your How many glucose molecules in 1 gram of sugar images are ready. How many glucose molecules in 1 gram of sugar are a topic that is being searched for and liked by netizens today. You can Get the How many glucose molecules in 1 gram of sugar files here. Find and Download all free photos and vectors.
If you’re searching for how many glucose molecules in 1 gram of sugar pictures information linked to the how many glucose molecules in 1 gram of sugar keyword, you have pay a visit to the right blog. Our site always gives you hints for seeking the maximum quality video and image content, please kindly surf and locate more enlightening video articles and graphics that fit your interests.
How Many Glucose Molecules In 1 Gram Of Sugar. One gram of carbohydrate yields 42 kilo calories of energy on respiration. One gram of table sugar is equal to 176 x 1021 molecules of sucrose. There are many different types of sugar but I will presume you mean glucose C6H12O6. This means that the cellulose molecule is straight and many such molecules can lay side by side in a parallel series of rows.
 Fructose Oses101 No Sugar Foods Simple Sugar Catching Monsters From pinterest.com
Fructose Oses101 No Sugar Foods Simple Sugar Catching Monsters From pinterest.com
Table 20-1 gives you the approximate effect of 1 gram glucose upon low blood sugar for various body weights. Putting this together we get. Of glucose molecules 001 x 6022 x 10 23 6022 x 10 21. The answer is 00055507486072617. The SI base unit for amount of substance is the mole. Converting backwards there are about 024 or nearly a quarter of a teaspoon of sugar in a gram of sugar.
Glucose has a molecular mass of 180 gmol.
How many molecules are in 1g of sugar. There are approximately 6 10 23 molecules per mole Avogadros number and as we said 24 atoms per molecule. The answer is 00055507486072617. Converting backwards there are about 024 or nearly a quarter of a teaspoon of sugar in a gram of sugar. Thats kind of a weird measurement but its actually the same as 1000 milligrams per liter or 1 gram per liter. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to. You can view more details on each measurement unit. There would be roughly 132 x 1022 glucose molecules. Each molecule of glucose produces 36 to 38 molecules of. How many molecules are in 1g of sugar.

Lets day 1 sugar cube weighs 4 grams. In this regard how much does a mole of glucose weigh. One gram of carbohydrate yields 42 kilo calories of energy on respiration. 612 121 116 180 grams. The answer is 00055507486072617.
 Source: researchgate.net
Source: researchgate.net
There are many different types of sugar but I will presume you mean glucose C6H12O6. This means that the cellulose molecule is straight and many such molecules can lay side by side in a parallel series of rows. Each molecule of glucose produces 36 to 38 molecules of. Note that rounding errors may occur so always check the results. So the molecular weight or weight of a mole of sugar is 180g.
 Source: sciencetrends.com
Source: sciencetrends.com
One gram of table sugar is equal to 176 x 1021 molecules of sucrose. Note that rounding errors may occur so always check the results. Salt NaCl Na 23 gramsfor each Na and Cl 35 grams for each Clapproximately Sugar let us assume it is glucose C6H12O6 C 12 grams per C and H 1 gram per H and O 16 grams per O. Then multiply that number by 6022 x 10²³ which is 1759x10²¹ molecules or. Table 20-1 gives you the approximate effect of 1 gram glucose upon low blood sugar for various body weights.
 Source: researchgate.net
Source: researchgate.net
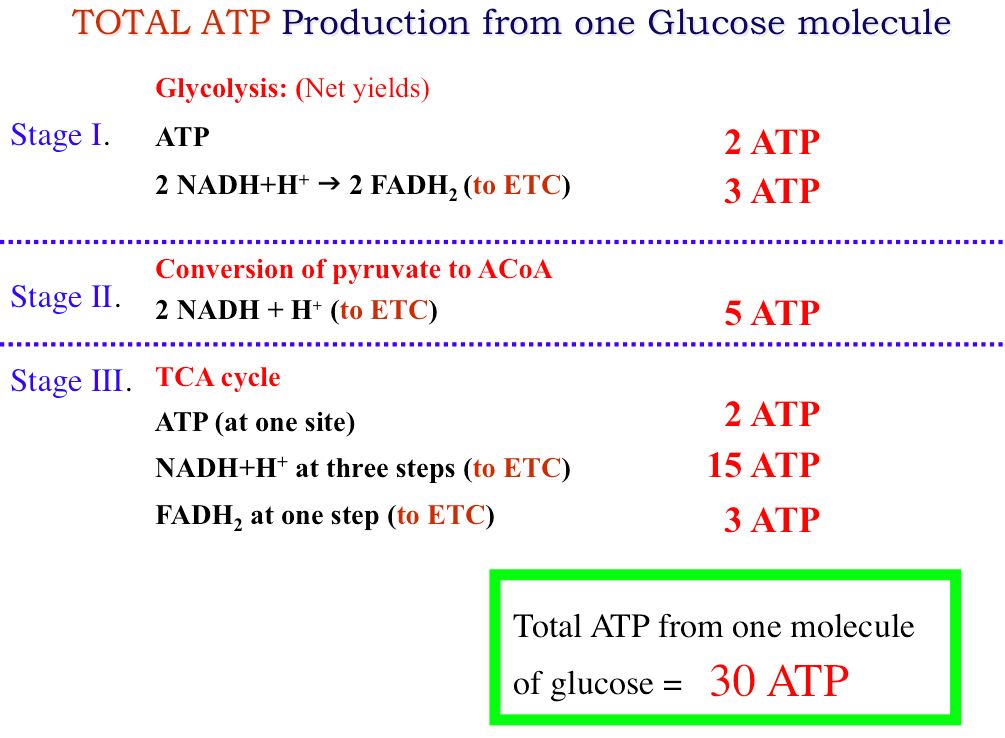
How many molecules of sucrose in that 1 liter of 05M sucrose solution. Biology textbooks often state that 38 ATP molecules can be made per oxidized glucose molecule during cellular respiration 2 from glycolysis 2 from the Krebs cycle and about 34 from the electron transport system. How many molecules are in 1g of sugar. The SI base unit for amount of substance is the mole. Remember that one mole of any substance contains Avogadro number of particles may be atoms or molecules or ions etc.
 Source: pinterest.com
Source: pinterest.com
If the substance is molecular the number molecules will be equal to 6022 x 10 23. Lets day 1 sugar cube weighs 4 grams. Assuming you are talking about table sugar sucrose which has a molar mass of 3422965gmol you would do 1000g divided by 3422965gmol to get the moles of sugar. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. If you are asking how many carbs are in 16 grams of sugar then your answer would be 16.
 Source: 3dchem.com
Source: 3dchem.com
In this regard how much does a mole of glucose weigh. Of glucose molecules 001 x 6022 x 10 23 6022 x 10 21. If the substance is molecular the number molecules will be equal to 6022 x 10 23. What is the molecular weight of glucose. Converting backwards there are about 024 or nearly a quarter of a teaspoon of sugar in a gram of sugar.
 Source: pinterest.com
Source: pinterest.com
One gram of table sugar is equal to 176 x 1021 molecules of sucrose. Use this page to learn how to. How many molecules of sucrose in that 1 liter of 05M sucrose solution. Biology textbooks often state that 38 ATP molecules can be made per oxidized glucose molecule during cellular respiration 2 from glycolysis 2 from the Krebs cycle and about 34 from the electron transport system. If the substance is molecular the number molecules will be equal to 6022 x 10 23.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. You can view more details on each measurement unit. N 8 10 25 atomskg. So the molecular weight or weight of a mole of sugar is 180g. How many moles of carbon are in C6H12O6.
 Source: alimentarium.org
Source: alimentarium.org
Your question as written isnt answerable as many of the unprocessed whole foods which are rich in carbohydrates do not contain much in the way of naturally occurring sugars. So the molecular weight or weight of a mole of sugar is 180g. Glucose has a molecular mass of 180 gmol. Your question as written isnt answerable as many of the unprocessed whole foods which are rich in carbohydrates do not contain much in the way of naturally occurring sugars. How many moles of carbon are in C6H12O6.
 Source: twinkl.fr
Source: twinkl.fr
05 moleliter x 1 liter x 6023x1023 moleculesmole 3012x1023 molecules same number of molecules How much of the 05M glucose solution is needed to provide 100 mg of glucose. If you are asking how many carbs are in 16 grams of sugar then your answer would be 16. The answer is 00055507486072617. 1 grams Glucose is equal to 00055507486072617 mole. The SI base unit for amount of substance is the mole.

Converting backwards there are about 024 or nearly a quarter of a teaspoon of sugar in a gram of sugar. So the molecular weight or weight of a mole of sugar is 180g. Remember that one mole of any substance contains Avogadro number of particles may be atoms or molecules or ions etc. Biology textbooks often state that 38 ATP molecules can be made per oxidized glucose molecule during cellular respiration 2 from glycolysis 2 from the Krebs cycle and about 34 from the electron transport system. Molecular weight of Glucose or grams The molecular formula for Glucose is C6H12O6.
 Source: biologystudynotes.blogspot.com
Source: biologystudynotes.blogspot.com
602 x 1023 molecules 401 x 1022 molecules of. Your question as written isnt answerable as many of the unprocessed whole foods which are rich in carbohydrates do not contain much in the way of naturally occurring sugars. Molecular weight of Glucose or grams The molecular formula for Glucose is C6H12O6. In above glucose sample the no. Convert 1st Grams to Moles then Moles to Molecules Molecules of Glucose 523 g Glucose x 1 mol Glucose1800 Glucose x 602 x 1023 molecules of Glucose1 mol Glucose 175 x 1022 molecules of Glucose Convert 1st Grams to Molecules then Molecules to Atoms Atoms O 175 x 1022 molecules Glucose 6 atoms O molecule Glucose 105 x 1023.

N 50 9 6 10 23 24 atomskg. What is the molecular weight of glucose. Remember that one mole of any substance contains Avogadro number of particles may be atoms or molecules or ions etc. 602 x 1023 molecules 401 x 1022 molecules of. Salt NaCl Na 23 gramsfor each Na and Cl 35 grams for each Clapproximately Sugar let us assume it is glucose C6H12O6 C 12 grams per C and H 1 gram per H and O 16 grams per O.
 Source: unm.edu
Source: unm.edu
How does that compare to the amount of solute in the 05M glucose solution. 612 121 116 180 grams. Biology textbooks often state that 38 ATP molecules can be made per oxidized glucose molecule during cellular respiration 2 from glycolysis 2 from the Krebs cycle and about 34 from the electron transport system. 602 x 1023 molecules 401 x 1022 molecules of. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many glucose molecules in 1 gram of sugar by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






