Your How many electrons in 1 mole of water images are available. How many electrons in 1 mole of water are a topic that is being searched for and liked by netizens today. You can Find and Download the How many electrons in 1 mole of water files here. Download all free photos.
If you’re looking for how many electrons in 1 mole of water pictures information linked to the how many electrons in 1 mole of water interest, you have visit the ideal site. Our website always provides you with suggestions for refferencing the highest quality video and picture content, please kindly surf and find more informative video content and graphics that match your interests.
How Many Electrons In 1 Mole Of Water. So 25 moles of water will have N25602310 231510 23 molecules. Thus in one molecule of water there are 10 electrons. So there will be a total of 602 1023 2 12 1024 hydrogen atoms. Since water has a chemical formula of H 2O there will be 2 moles of hydrogen in every mole of water.
 I Calculate The Total Number Of Electrons Present In 1 Mole Of Methane Ii Find A The Tot Youtube From youtube.com
I Calculate The Total Number Of Electrons Present In 1 Mole Of Methane Ii Find A The Tot Youtube From youtube.com
One mole of water contains 602 x 10 23 MOLECULES of water. Recall that 1 mole is around 6022 10²³ molecules ie. So 9g of water will be half of a mole. How many moles equal a gram. So 30 g of water is the mass of approximately 100285 10²⁴ molecules of water. 0 2 2 1 0 2 3 6.
Of electrons in 1 m o l e of O 2 1 0 6.
The given are. Correct option is D As we know one mole of each substance contains N A. 1 mole of electrons contains the Avogadro constant L electrons that is 602 x 1023 electrons. So in one mole of water there are 10 moles of electrons. Gain of oxygen d. Loss of hydrogen c.
 Source: pinterest.com
Source: pinterest.com
Thus 1 kg of water or ice has 5555 moles of H20. Each molecule of water has 8 11 10 electrons. 227 mol now number of electrons in CO2 are obtained by adding total electrons in each of the three atoms ie. Recall that 1 mole is around 6022 10²³ molecules ie. In one mole of water there will exist approximately 602 1023 water molecules.
 Source: youtube.com
Source: youtube.com
Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. Thus in one molecule of water there are 10 electrons. So 25 moles of water will have N25602310 231510 23 molecules. One mole of water contains 602 x 10 23 MOLECULES of water. Calculate the number of moles of H 2.
 Source: pinterest.com
Source: pinterest.com
How many moles will be there in 1 kg of water. Of electrons in 1 m o l e of O 2 1 0 6. 1 mole of any substance contains 6 X 10 23 molecules. 0 2 2 1 0 2 3 6. Correct option is D As we know one mole of each substance contains N A.
 Source: clutchprep.com
Source: clutchprep.com
4 e- 4 H 2 Ol 2 H 2 g 4 OH-aq Calculate the number of moles of electrons. Electrons 602310 2310602310 24. 30 g of water is the mass of 30 g 118015 molg 166528 mol. Mass moles of water moles of electrons. So half a mole will have about 3 X 10 23 molecules.
 Source: pinterest.com
Source: pinterest.com
Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. An atom of hydrogen has 1 electron an atom of oxygen has 8 electrons. So there will be a total of 602 1023 2 12 1024 hydrogen atoms. So half a mole will have about 3 X 10 23 molecules. The number of atoms is triple.
 Source: pinterest.com
Source: pinterest.com
So 25 moles of water will have N25602310 231510 23 molecules. A plasma must contain many what. How many moles equal a gram. Hydrogen is produced during the reduction of water at the cathode. How many molecules are present in one mole of water H2O.

So 9g of water will be half of a mole. How many molecules are present in one mole of water H2O. So there will be a total of 602 1023 2 12 1024 hydrogen atoms. Izvoru47 and 6 more users found this answer helpful. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place.
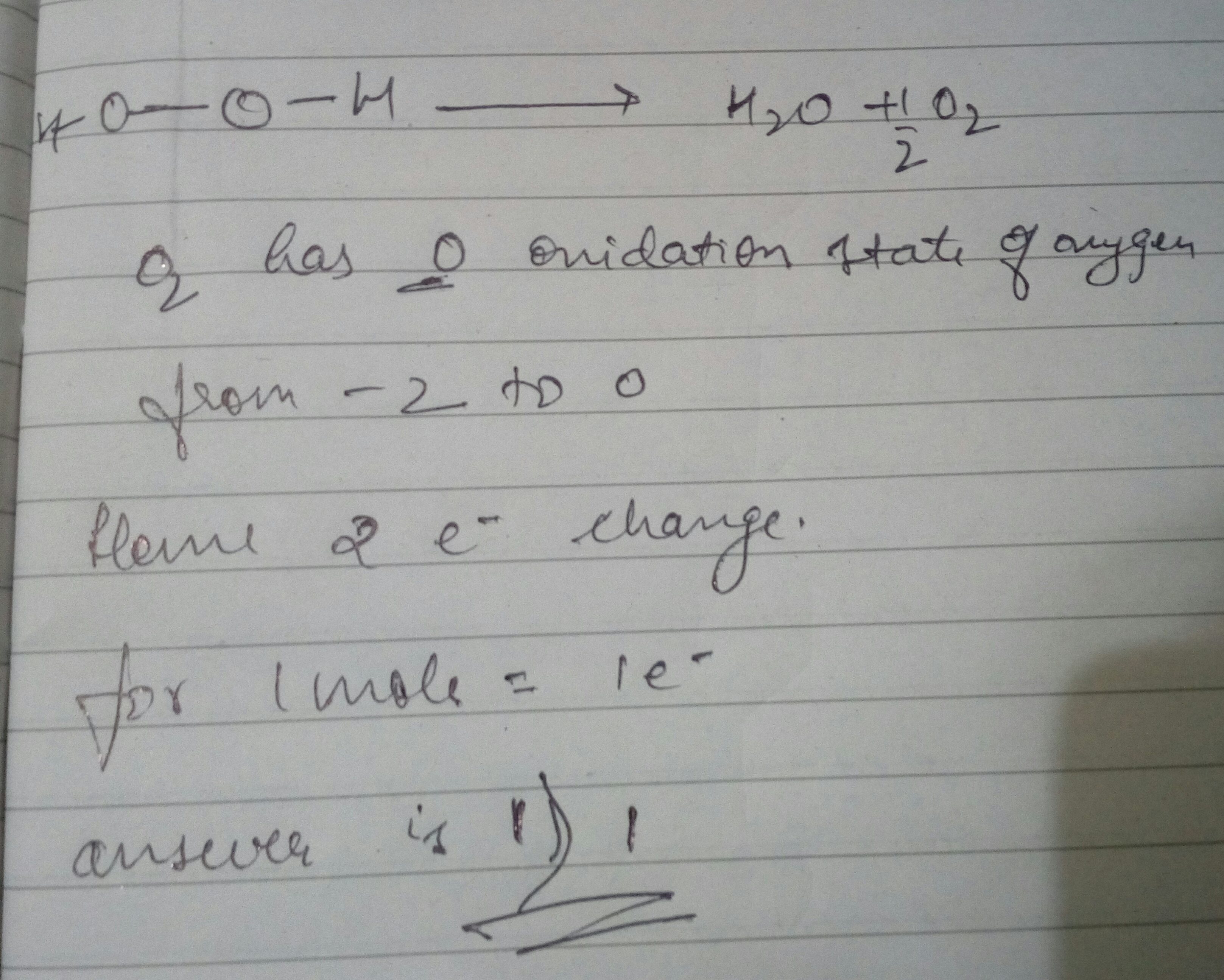 Source: embibe.com
Source: embibe.com
Then water H₂O has a total molar mass of about 18015 gmol and has 10 electrons. This comes out be 5555 moles. An atom of hydrogen has 1 electron an atom of oxygen has 8 electrons. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. Hydrogen is produced during the reduction of water at the cathode.
 Source: pinterest.com
Source: pinterest.com
In one mole of water there will exist approximately 602 1023 water molecules. Waters chemical structure is H2O which means 2 atoms of hydrogen and one atom of oxygen. 18 ml means 18 g. One mole of water contain 6022 140 85774 molecules. The number of atoms is triple.
 Source: pinterest.com
Source: pinterest.com
A plasma must contain many what. Mass moles of water moles of electrons. How many moles equal a gram. If you have a compound like H 2 O then. Electrons 602310 2310602310 24.
 Source: youtube.com
Source: youtube.com
So half a mole will have about 3 X 10 23 molecules. 96 500 Coulombs 1 mole and 1 mole 602xx1023 electrons And to determine the number of Coulombs in 100 electrons use the method of converting one unit to another unit. 4 e- 4 H 2 Ol 2 H 2 g 4 OH-aq Calculate the number of moles of electrons. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. Loss of electrons b.
 Source: askiitians.com
Source: askiitians.com
30 g of water is the mass of 30 g 118015 molg 166528 mol. Loss of electrons b. Two O and one C 688 22 thus one mole of CO2 has 22 6022 1023 electrons and 227 mol has 227 22 6022 1023 electrons 3011 1023 electrons. Waters chemical structure is H2O which means 2 atoms of hydrogen and one atom of oxygen. Calculate the number of moles of H 2.
 Source: pinterest.com
Source: pinterest.com
Correct option is A Hint. Hydrogen is produced during the reduction of water at the cathode. How many molecules are present in one mole of glucose C6H12O6. 227 mol now number of electrons in CO2 are obtained by adding total electrons in each of the three atoms ie. You would also be given that in an exam if you needed to use it.

Each molecule of water has 8 11 10 electrons. Loss of electrons b. 18 ml means 18 g. 30 g of water is the mass of 30 g 118015 molg 166528 mol. One molecule of water has 10 electrons.
 Source: pinterest.com
Source: pinterest.com
You would also be given that in an exam if you needed to use it. A Plasma must contain many ions and electrons. A plasma must contain many what. 30 g of water is the mass of 30 g 118015 molg 166528 mol. Just like there are ten electrons per molecules of water there are ten moles of electrons per each mole of water so just multiplying number of moles of water by ten will give the correct answer in other words.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many electrons in 1 mole of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






