Your How many carbon atoms in 1 mole of co2 images are ready. How many carbon atoms in 1 mole of co2 are a topic that is being searched for and liked by netizens now. You can Find and Download the How many carbon atoms in 1 mole of co2 files here. Download all free images.
If you’re searching for how many carbon atoms in 1 mole of co2 pictures information connected with to the how many carbon atoms in 1 mole of co2 keyword, you have come to the ideal site. Our site frequently gives you suggestions for refferencing the maximum quality video and picture content, please kindly search and find more informative video articles and graphics that match your interests.
How Many Carbon Atoms In 1 Mole Of Co2. Therefore 250 mole of CO2 will contain 4521024 atoms. 250L x 1 mol224 L 0112 moles CO 2. 2 mole of oxygen atoms per mole of oxygen atoms 2 x 602 x 1023 oxygen atoms. Similarly how many atoms are in 3 moles of co2.

So 3 moles of CO2 contains 3 x. A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms. Each nitrate ion contains one nitrogen atom and three oxygen atoms. So to find the numb of O atoms you do the following. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. 1 molecule of CO2 contains 3 atoms so.
The formula of carbon dioxide is CO2.
A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. The chemical formula for carbon dioxide CO₂ indicates that there are three moles of atoms in one mole of the compound. 1 mole carbon dioxide contains 602 x 1023 molecules. One mole of C 60210 23 atoms of carbon. 1 Mole is 6021023 molecules.
 Source: sites.google.com
Source: sites.google.com
In one mole of carbon there are 602 1023 atoms. Now in mol of CO2 there are 6022x10 23 molecules and in each molecule there are 2 atoms of O. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. The atom ratio and the mole ratio of the elements are identical. Therefore the answer is 3 atoms x 4 moles x 602214179 x 1023 atomsmole 7226570148 x 1023 atoms.
 Source: slideplayer.com
Source: slideplayer.com
There are 6022 X 1023 atoms of carbon in 1 mole of carbon. One molecule of CO2 contains one atom of carbon and two atoms of oxygen One mole of CO2 contains one mole of carbon atoms and two moles of oxygen atoms. What is the mass of 004 mole of CO2. The chemical formula for carbon dioxide CO₂ indicates that there are three moles of atoms in one mole of the compound. Lets consider the carbon dioxide molecule.
 Source: slideserve.com
Source: slideserve.com
The atom ratio and the mole ratio of the elements are identical. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. One mole of CO 2. The value of the mole in precisely 12 grammes of pure carbon-12 is equal to the number of atoms. 1511024 molecule of CO2 will contain 4521024 atoms.
 Source: slideplayer.com
Source: slideplayer.com
1 mole carbon dioxide contains 602 x 1023 molecules. Therefore there are 6022 x 1023 atoms of carbon and 12044 x. Therefore the answer is 3 atoms x 4 moles x 602214179 x 1023 atomsmole 7226570148 x 1023 atoms. Two moles of O. 250L x 1 mol224 L 0112 moles CO 2.

1 mole carbon dioxide contains 602 x 1023 molecules. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. So 3 moles of CO2 contains 3 x. Correct option is B One mole of CO 2. 1 mole carbon dioxide contains 602 x 1023 molecules.

Each nitrate ion contains one nitrogen atom and three oxygen atoms. Two moles of O. Calculate the moles of CO₂ from its mass and molar mass. To obtain the number of atoms just use the the formula nNLA. So to find the numb of O atoms you do the following.
 Source: pinterest.com
Source: pinterest.com
1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. Lets consider the carbon dioxide molecule. Avogadros number shows us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gas. Each nitrate ion contains one nitrogen atom and three oxygen atoms. Therefore there are 6022 x 1023 atoms of carbon and 12044 x 1023 atoms of oxygen in that 1 mole of CO2.
 Source: youtube.com
Source: youtube.com
A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms. Hence 5 mole of carbon dioxide consists of 301 x1024 molecules. Two moles of O. The atom ratio and the mole ratio of the elements are identical. The unit for the mole is mol just like the unit for the dozen is doz.

Therefore 3 Moles of Carbon Dioxide CO2 3 6021023 or 18061023 molecules of CO2. Therefore the answer is 3 atoms x 4 moles x 602214179 x 1023 atomsmole 7226570148 x 1023 atoms. 1 mole carbon dioxide contains 602 x 1023 molecules. How many atoms are there in one mole of carbon dioxide. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms.

So in two moles there will be twice that. So in two moles there will be twice that. 1511024 molecule of CO2 will contain 4521024 atoms. 3 atoms 18061023 molecules 54181023 atoms. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms.
 Source: slideplayer.com
Source: slideplayer.com
Lets consider the carbon dioxide molecule. We know it has the formula CO2 and this tells us that. How many atoms are there in one mole of carbon dioxide. What is the mass of 004 mole of CO2. Related Links How Many Different Words Can Be Formed By Jumbling The Letters In The Word Mississippi.
 Source: slideplayer.com
Source: slideplayer.com
A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms. In one mole of carbon there are 602 1023 atoms. 2 mole of oxygen atoms per mole of oxygen atoms 2 x 602 x 1023 oxygen atoms. One mole of CO 2. Avogadros number shows us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gas.
 Source: slideplayer.com
Source: slideplayer.com
Related Links How Many Different Words Can Be Formed By Jumbling The Letters In The Word Mississippi. Two moles of O. A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms. Now in mol of CO2 there are 6022x10 23 molecules and in each molecule there are 2 atoms of O. A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. Correct option is B One mole of CO 2. A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. The atom ratio and the mole ratio of the elements are identical.

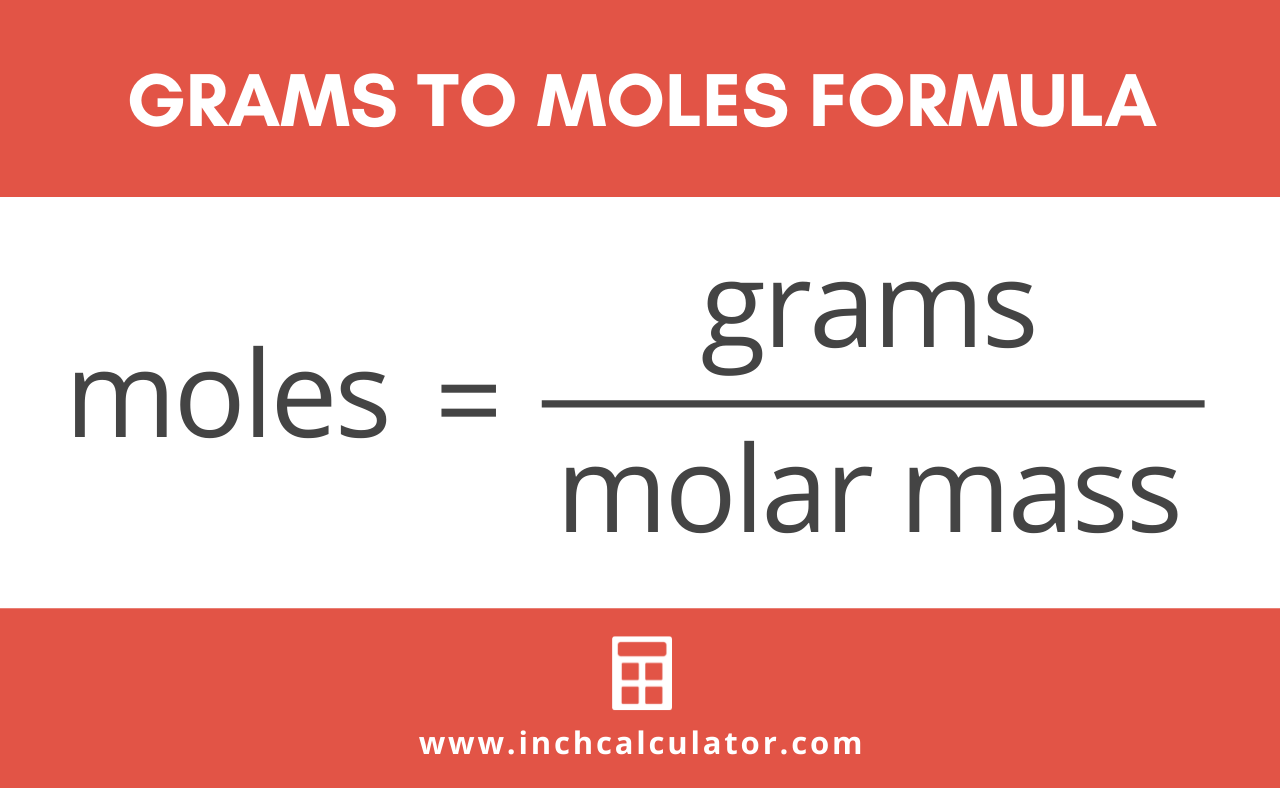
Lets consider the carbon dioxide molecule. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. The value of the mole in precisely 12 grammes of pure carbon-12 is equal to the number of atoms. Similarly how many atoms are in 3 moles of co2. 05 g CO₂ 1mol CO₂44g CO₂ 00113636 mol CO₂.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many carbon atoms in 1 mole of co2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.